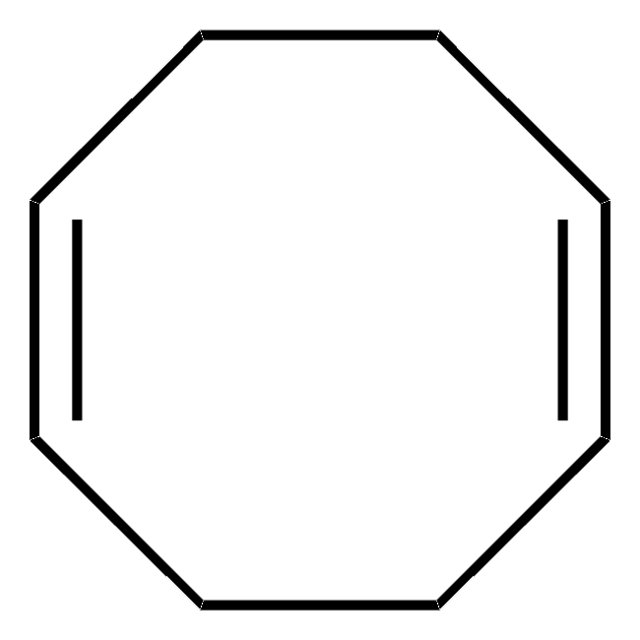
推薦產品
蒸汽壓力
25.8 mmHg ( 37.7 °C)
6.8 mmHg ( 25 °C)
化驗
≥99%
形狀
liquid
自燃溫度
431 °F
純化經由
redistillation
不包含
stabilizer
折射率
n20/D 1.493 (lit.)
bp
149-150 °C (lit.)
mp
−69 °C (lit.)
密度
0.882 g/mL at 25 °C (lit.)
SMILES 字串
C1CC=CCCC=C1
InChI
1S/C8H12/c1-2-4-6-8-7-5-3-1/h1-2,7-8H,3-6H2/b2-1-,8-7-
InChI 密鑰
VYXHVRARDIDEHS-QGTKBVGQSA-N
尋找類似的產品? 前往 產品比較指南
應用
1,5-环辛二烯被用作合成氨基茚衍生物的催化剂,通过酮亚胺与内部和末端炔烃的环合反应。
訊號詞
Danger
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
100.4 °F - closed cup
閃點(°C)
38 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
Midori Nagamoto et al.
Chemical communications (Cambridge, England), 50(47), 6274-6277 (2014-05-07)
[3 + 2] Annulation of ketimines with internal and terminal alkynes proceeded via C-H activation to give aminoindene derivatives in high yields, which is catalyzed by a cationic iridium complex coordinated with 1,5-cyclooctadiene (cod).
Eleonora Cavallari et al.
The journal of physical chemistry. B, 119(31), 10035-10041 (2015-07-15)
Hyperpolarization of (13)C carboxylate signals of metabolically relevant molecules, such as acetate and pyruvate, was recently obtained by means of ParaHydrogen Induced Polarization by Side Arm Hydrogenation (PHIP-SAH). This method relies on functionalization of the carboxylic acid with an unsaturated
Adrian Tlahuext-Aca et al.
Dalton transactions (Cambridge, England : 2003), 43(42), 15997-16005 (2014-09-19)
Ni(0)-catalyzed dehydrogenation of benzylic-type imines was performed to yield asymmetrical tetra-substituted imidazoles and 2-imidazolines. This was achieved with a single operational step while maintaining good selectivity and atom economy. The catalytic system shows low to moderate tolerance for fluoro-, trifluoromethyl-
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務