全部照片(1)
About This Item
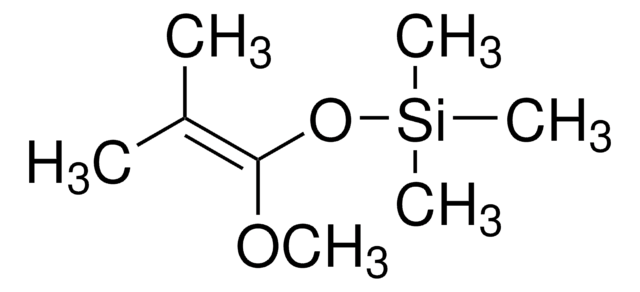
線性公式:
C6H5C(=CH2)OSi(CH3)3
CAS號碼:
分子量::
192.33
Beilstein:
1306914
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
蒸汽密度
>1 (vs air)
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.502 (lit.)
bp
88-89 °C/11 mmHg (lit.)
密度
0.938 g/mL at 25 °C (lit.)
官能基
phenyl
儲存溫度
2-8°C
SMILES 字串
C[Si](C)(C)OC(=C)c1ccccc1
InChI
1S/C11H16OSi/c1-10(12-13(2,3)4)11-8-6-5-7-9-11/h5-9H,1H2,2-4H3
InChI 密鑰
AFFPCIMDERUIST-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
1-Phenyl-1-trimethylsiloxyethylene is a styrene type silyl enol ether, reacts with formaldehyde and 2,4-pentanedione to yield the corresponding dihydropyran[1]. It also undergoes Mukaiyama aldol reaction with aldehydes in water in the presence of amphiphilic calix[6]arene derivatives as surfactants[2].
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
168.8 °F - closed cup
閃點(°C)
76 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
客戶也查看了
Calix [6] arene derivatives bearing sulfonate and alkyl groups as surfactants in Sc (OTf)3-catalyzed Mukaiyama aldol reactions in water.
Tian H-Y, et al.
Tetrahedron Letters, 41(15), 2529-2532 (2000)
Novel one-pot Mannich-type reaction in water: indium trichloride-catalyzed condensation of aldehydes, amines and silyl enol ethers for the synthesis of ?-amino ketones and esters.
Loh T-P and Wei L-L.
Tetrahedron Letters, 39(3), 323-326 (1998)
Robert J Hinkle et al.
Tetrahedron, 65(34), 6834-6839 (2010-02-18)
The rapid synthesis of cis-2,6-disubstituted dihydropyrans is achieved in a three-component, one-pot cascade reaction. BiBr(3)-ediated addition of ketene silyl acetals or silyl enol ethers to beta,gamma-unsaturated cis-4-trimethylsilyl-3-butenal provides a Mukaiyama aldol adduct containing a vinylsilane moiety tethered to a silyl
Catalyst-free aqueous multicomponent domino reactions from formaldehyde and 1, 3-dicarbonyl derivatives.
Gu Y, et al.
Green Chemistry, 11(12), 1968-1972 (2009)
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務