推薦產品
品質等級
化驗
97%
形狀
solid
mp
270-273 °C (lit.)
SMILES 字串
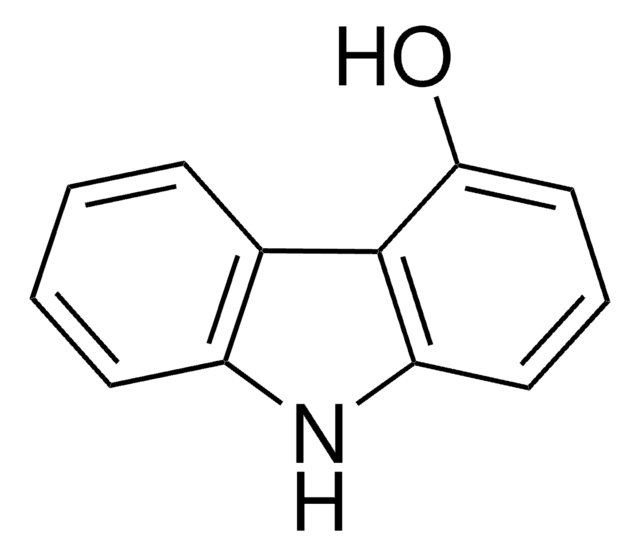
Oc1ccc2c(c1)[nH]c3ccccc23
InChI
1S/C12H9NO/c14-8-5-6-10-9-3-1-2-4-11(9)13-12(10)7-8/h1-7,13-14H
InChI 密鑰
GWPGDZPXOZATKL-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2-Hydroxycarbazole is a compound structurally related to the Ca2+-mobilizing marine toxin, 9-methyl-7-bromoeudistomin[1]. Room temperature electronic absorption and fluorescence spectra of 2-hydroxycarbazole has been studied in concentrated aqueous potassium hydroxide solutions[2]. It undergoes chemoselective N-alkylation using NaH as a base in a THF-DMF solvent system[3].
應用
2-Hydroxycarbazole was used in the synthesis of isochromene fused carbazol, (4aS,13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole[4].
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Nguyen Manh Cuong et al.
Natural product communications, 4(7), 921-924 (2009-09-08)
The first synthesis of isochromene fused carbazols, (4aS, 13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole (2) and its epi-isomer 3 by condensation of citral and 2-hydroxycarbazole using Ti(OEt)4 and MeAlC12 as catalysts is described.
Tamanna Mallick et al.
Colloids and surfaces. B, Biointerfaces, 172, 440-450 (2018-09-10)
Six structurally different carbazoles (1-6) were explored as the green reducing agents for the synthesis of the fluorescent Au nanoparticles with tailor-made morphology in anionic (sodium dodecyl sulphate, SDS), cationic (cetyltrimethylammonium bromide, CTAB) and neutral (polyvinylpyrrolidone, PVP) micelle medium. Structure
K Zawadzka et al.
Environmental science and pollution research international, 22(24), 19658-19666 (2015-08-16)
Nitrogen heterocyclic compounds, especially carbazole, quinolone, and pyridine are common types of environmental pollutants. Carbazole has a toxic influence on living organisms, and the knowledge of its persistence and bioconversion in ecosystems is still not complete. There is an increasing
Chemoselective N-alkylation of 2-hydroxycarbazole as a model for the synthesis of N-substituted pyrrole derivatives containing acidic functions.
Albanese D, et al.
Tetrahedron, 51(19), 5681-5688 (1995)
J M Thomas et al.
The Journal of pharmacology and experimental therapeutics, 298(2), 644-650 (2001-07-17)
2-hydroxycarbazole, a compound structurally related to the Ca2+-mobilizing marine toxin 9-methyl-7-bromoeudistomin, has recently been proposed to activate both type 1 and type 2 ryanodine receptors in skeletal and cardiac muscle, respectively. This study was undertaken to evaluate the activity of
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務