推薦產品
化驗
97%
形狀
solid
mp
~265 °C (dec.) (lit.)
溶解度
methanol:glacial acetic acid (1:1): soluble 25 mg/mL, clear, colorless to light yellow
SMILES 字串
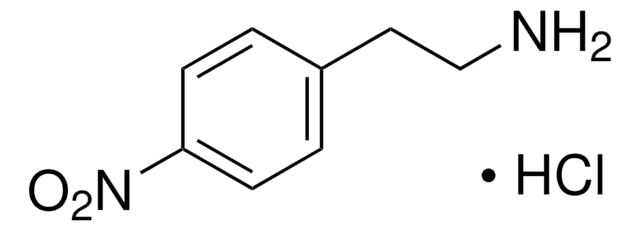
Cl.NCc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H8N2O2.ClH/c8-5-6-1-3-7(4-2-6)9(10)11;/h1-4H,5,8H2;1H
InChI 密鑰
SMIXZZMSWYOQPW-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
應用
4-Nitrobenzylamine hydrochloride was used in chemical modification of graphite powder and multiwalled carbon nanotubes. It was also used in the preparation of 2-fluoro-6-(4-nitrohenzylamino)purine.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
客戶也查看了
A Z Kirkel' et al.
Voprosy meditsinskoi khimii, 32(2), 118-125 (1986-03-01)
Oxidation of p-nitro- and p-dimethylaminomethyl derivatives of benzylamine, catalyzed by amine oxidases from human placenta and blood serum, was studied. The amine oxidase activity was estimated by means of a spectrophotometric procedure involving measurement of aldehyde formed during the reaction
Gregory G Wildgoose et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 6(2), 352-362 (2005-03-09)
We demonstrate that graphite powder and multiwalled carbon nanotubes (MWCNTs) can be derivatised by 4-nitrobenzylamine (4-NBA) simply by stirring the graphite powder or MWCNTs in a solution of acetonitrile containing 10 mM 4-NBA. We propose that 4-NBA partially intercalates at
Combinatorial synthesis of 2, 9-substituted purines.
Gray NS, et al.
Tetrahedron Letters, 38(7), 1161-1164 (1997)
M A Akyüz et al.
Journal of neural transmission (Vienna, Austria : 1996), 114(6), 693-698 (2007-04-03)
Computational studies using the ONIOM methods have been performed to probe the catalytic roles of tyrosine residues 398 and 435 which constitute the "aromatic cage" in the active site of MAO-B. The results presented here provide additional new insights into
The synthesis of a light-switchable amino acid for inclusion into conformationally mobile peptides.
Ulysse L and Chmielewski J.
Bioorganic & Medicinal Chemistry Letters, 4(17), 2145-2146 (1994)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務