全部照片(1)
About This Item
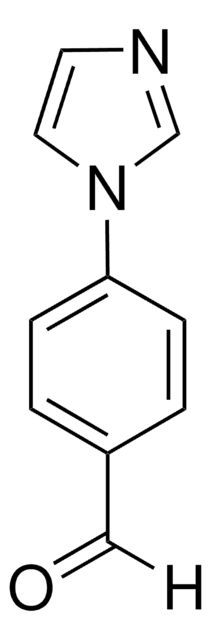
經驗公式(希爾表示法):
C11H10N2O
CAS號碼:
分子量::
186.21
EC號碼:
MDL號碼:
分類程式碼代碼:
12352005
PubChem物質ID:
NACRES:
NA.22
暫時無法取得訂價和供貨情況
推薦產品
化驗
96%
形狀
solid
mp
108-110 °C (lit.)
官能基
ketone
SMILES 字串
CC(=O)c1ccc(cc1)-n2ccnc2
InChI
1S/C11H10N2O/c1-9(14)10-2-4-11(5-3-10)13-7-6-12-8-13/h2-8H,1H3
InChI 密鑰
GAIQQJIMVVUTQN-UHFFFAOYSA-N
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
J Triscari et al.
International journal of obesity, 11 Suppl 3, 43-51 (1987-01-01)
A selective inhibitor of thromboxane synthase, Ro 22-3581 has been shown to be a useful tool for investigating the relationship between hyperinsulinemia and obesity. These studies have established that the pharmacologic normalization of the hyperinsulinemia associated with elevated weights in
A J Watson et al.
American journal of kidney diseases : the official journal of the National Kidney Foundation, 8(1), 26-30 (1986-07-01)
It has recently been postulated that thromboxane A2 may participate in the pathogenesis of acute myohemoglobinuric experimental acute renal failure. To investigate this further, the effect of selective inhibition of thromboxane synthesis on the course of glycerol-induced acute renal failure
W D Watkins et al.
Prostaglandins, 23(3), 273-285 (1982-03-01)
We assessed the effect of a specific thromboxane synthetase inhibitor (an imidazole derivative) on pulmonary hemodynamics and the concentrations of TxB2 (TxA2), 6-keto-PGF1 alpha (PGI2), and PGF2 in pulmonary lymph and transpulmonary blood samples following intravenous administration of E. coli
H D Uderman et al.
Prostaglandins, 24(2), 237-244 (1982-08-01)
The compound 4'-(imidazol-1-yl) acetophenone was demonstrated to be a selective thromboxane (Tx) synthetase inhibitor in spontaneously hypertensive rats (SHR). Serum TxB2 concentrations (from clotted blood) were suppressed by 89.1% (p less than 0.001) and 41.2% (p less than 0.01) at
Viatcheslav Stepanenko et al.
Tetrahedron, asymmetry, 18(23), 2738-2745 (2007-11-26)
The effectiveness of several spiroborate ester catalysts was investigated in the asymmetric borane reduction of 2-, 3-, 4-acetylpyridines under different reaction conditions. Highly enantiomerically enriched 1-(2-, 3- and 4-pyridyl)ethanols and 1-(heterocyclic)ethanols were obtained using 1 to 10% catalytic loads of
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務