推薦產品
化驗
96.0%
mp
163-165 °C (lit.)
溶解度
methanol: soluble 5%, clear to slightly hazy (colorlessto faint brown)
SMILES 字串
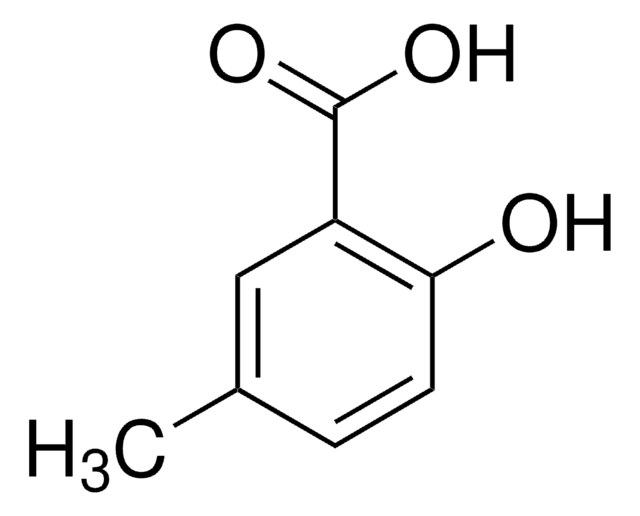
Cc1cccc(C(O)=O)c1O
InChI
1S/C8H8O3/c1-5-3-2-4-6(7(5)9)8(10)11/h2-4,9H,1H3,(H,10,11)
InChI 密鑰
WHSXTWFYRGOBGO-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
客戶也查看了
Krithika Delhiraja et al.
Environmental monitoring and assessment, 192(7), 432-432 (2020-06-17)
Emerging contaminants (ECs) have become an increasing area of concern due to the likely impacts of these compounds on human health and the environment. Generally, products which are used for households and personal care activities contribute to major percentage of
Synthesis of (R)-, (S)-, and (RS)-hydroxymethylmexiletine, one of the major metabolites of mexiletine.
Cavalluzzi MM, et al.
Tetrahedron Asymmetry, 18(20), 2409-2417 (2007)
Generalized Method for the Production of 1, 3-Benzoxazine, 1, 3-Benzothiazine, and Quinazoline Derivatives from 2-(Hydroxy, Thio, or Amino) Aromatic Acids Using Triphenylphosphine Thiocyanogen.
Pritchard KM, et al.
Synthetic Communications, 35(!2), 1601-1611 (2005)
On structure-related properties of synthetic organic clot-dissolving (thrombolytic) compounds.
K N von Kaulla et al.
Biochemical pharmacology, 16(6), 1023-1034 (1967-06-01)
Warren B Cross et al.
Dalton transactions (Cambridge, England : 2003), (7)(7), 1287-1293 (2005-03-23)
Tungsten(VI) oxo-salicylate complexes were prepared in moderate yield (47 to 63%) by the reactions of WOCl4 and two equivalents of either 3-methylsalicylic acid (MesaliH2) or 3,5-di-isopropylsalicylic acid (di-i-PrsaliH2). Performing the reaction in refluxing toluene afforded the two analogous ditungsten complexes
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務