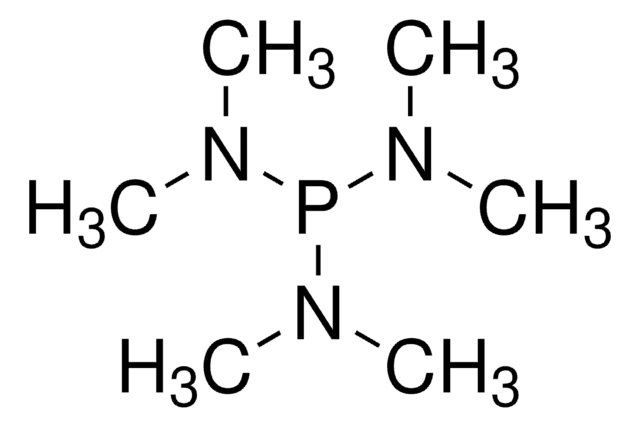
推薦產品
品質等級
化驗
96%
環保替代產品評分
old score: 22
new score: 3
Find out more about DOZN™ Scoring
環保替代產品特色
Atom Economy
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
84-86 °C (lit.)
官能基
carboxylic acid
phenyl
環保替代類別
SMILES 字串
OC(=O)C\C=C\c1ccccc1
InChI
1S/C10H10O2/c11-10(12)8-4-7-9-5-2-1-3-6-9/h1-7H,8H2,(H,11,12)/b7-4+
InChI 密鑰
PSCXFXNEYIHJST-QPJJXVBHSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
已对反式苯乙烯乙酸氢键系统的偏振红外光谱进行研究[1]。
我们竭诚为您带来满足绿色替代产品四大类别要求的替代产品。本品属于重新设计产品类别,在“原子经济”、“设计要有能效”和“使用可再生的原料”绿色化学原则方面取得了重大进步。 点击此处查看其DOZN记分卡。
應用
反式苯乙烯乙酸(4-苯基-3-丁烯酸)作为基于机制的肽酰甘氨酸 α 抑制剂-羟基化单加氧酶 [2]。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
In vivo inhibition of peptidylglycine-alpha-hydroxylating monooxygenase by 4-phenyl-3-butenoic acid.
G P Mueller et al.
The Journal of pharmacology and experimental therapeutics, 290(3), 1331-1336 (1999-08-24)
Peptidylglycine-alpha-hydroxylating monooxygenase (PHM; EC 1.14.17. 3) catalyzes the first and rate-limiting reaction in the two-step process that alpha-amidates neural and endocrine peptides. The substrate analog 4-phenyl-3-butenoic acid (PBA) was shown in vitro to selectively inhibit PHM without affecting the activity
Jee Yeon Lee et al.
Journal of agricultural and food chemistry, 53(20), 7696-7700 (2005-09-30)
An antifungal compound was isolated from the culture broth of Streptomyces koyangensis strain VK-A60 using various chromatographic procedures. On the basis of the high-resolution EI-mass and 1H and 13C NMR data, the compound was identified as 4-phenyl-3-butenoic acid. Colletotrichum orbiculare
G A Abou-Mohamed et al.
Journal of cardiovascular pharmacology, 35(6), 871-880 (2000-06-03)
Formation of mature active neuropeptides such as substance P (SP) from their glycine extended precursors entails alpha-amidation of peptide precursors by the sequential enzymatic action of peptidylglycine alpha-monooxygenase (PAM) and peptidylamidoglycolate lyase (PGL). We reported that these two enzymes that
Jee Yeon Lee et al.
International journal of systematic and evolutionary microbiology, 55(Pt 1), 257-262 (2005-01-18)
A 4-phenyl-3-butenoic acid-producing actinomycete, designated strain VK-A60T, was isolated from a soil sample collected from Koyang, Korea. Morphological and chemical characteristics of the strain were consistent with those of the genus Streptomyces. The cell wall of the strain contains LL-diaminopimelic
W J Driscoll et al.
Biochemistry, 39(27), 8007-8016 (2000-07-13)
The bifunctional enzyme peptidylglycine-alpha-amidating monooxygenase mediates the conversion of C-terminal glycine-extended peptides to their active alpha-amidated products. Peptidylglycine-alpha-hydroxylating monooxygenase (PHM, EC 1.14.17. 3) catalyzes the first reaction in this two-step process. The olefinic compound 4-phenyl-3-butenoic acid (PBA) is the most
Active Filters
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務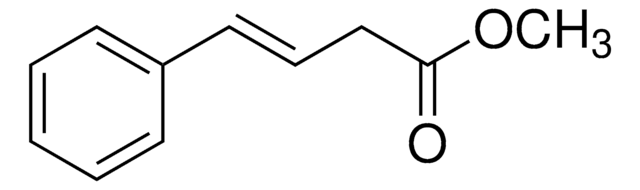



![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)