推薦產品
化驗
97%
mp
75-77 °C (lit.)
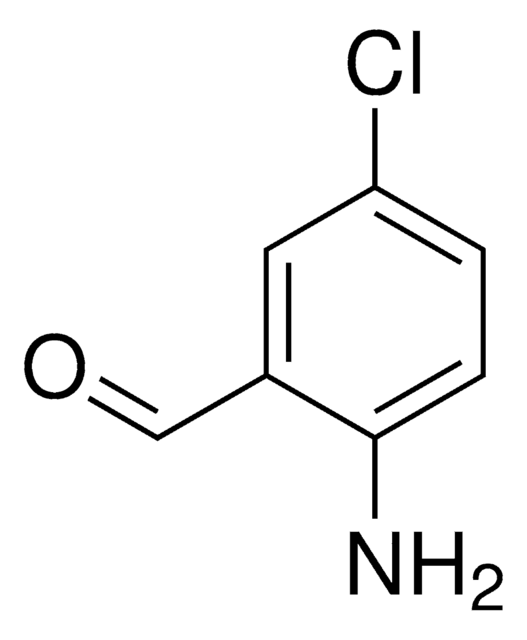
SMILES 字串
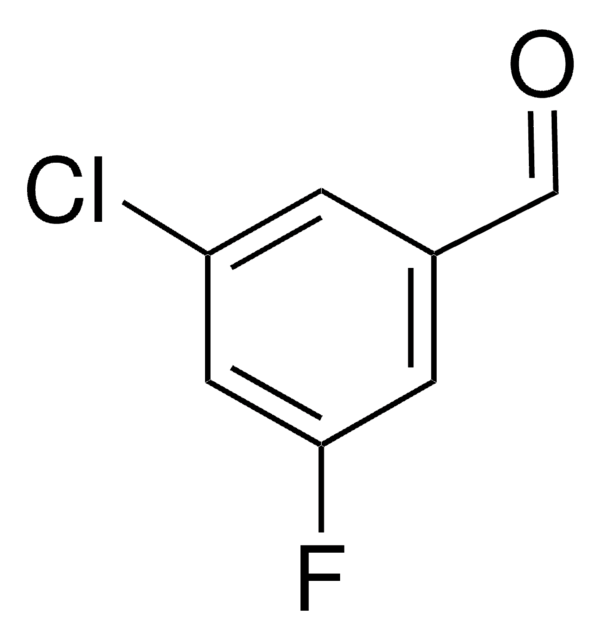
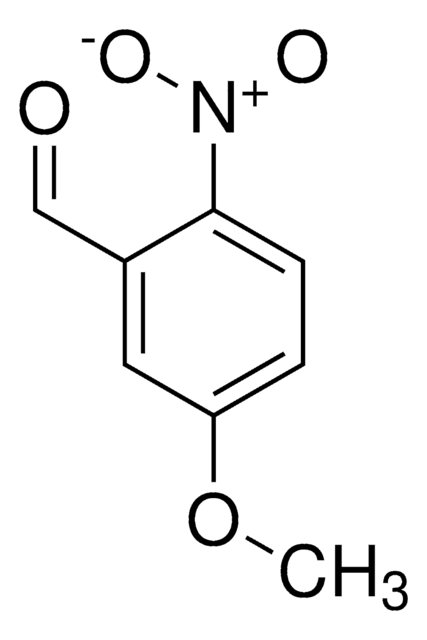
[H]C(=O)c1cc(ccc1Cl)[N+]([O-])=O
InChI
1S/C7H4ClNO3/c8-7-2-1-6(9(11)12)3-5(7)4-10/h1-4H
InChI 密鑰
VFVHWCKUHAEDMY-UHFFFAOYSA-N
尋找類似的產品? 前往 產品比較指南
一般說明
2-Chloro-5-nitrobenzaldehyde undergoes condensation reaction with 2-methyl-1-propenylbenzimidazole to yield (E,Z)-2-(2-chloro-5-nitrostyryl)-1-(1-propenyl)benzimidazole. It reacts with 5-aminopyrazoles to yield symmetrical bispyrazolo[3,4-]pyridines.
應用
2-Chloro-5-nitrobenzaldehyde was used in the synthesis of 5-nitro-2-(1H-pyrrol-1-yl)benzaldehyde.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
D E Bacelo et al.
Acta crystallographica. Section C, Crystal structure communications, 53 ( Pt 7), 907-909 (1997-07-15)
The title compound, C18H14ClN3O2, was synthesized by the condensation of 2-chloro-5-nitrobenzaldehyde with 2-methyl-1-propenylbenzimidazole, and the molecule comprises a 2-chloro-5-nitrobenzene and a 1-(Z)-propenylbenzimidazole. The two aromatic moieties are conjugated through the vinyl group. The dihedral angle between the two rings is
Reactions of 5-amino-1, 2-azoles with aromatic and heterocyclic o-chloroaldehydes:[1+ 1] versus [2+ 1] cyclocondensation.
Abramov MA, et al.
Tetrahedron, 57(44), 9123-9129 (2001)
Morita-Baylis-Hillman route to 4H-pyrrolo [1, 2-a][1] benzazepine derivatives.
Park SP, et al.
Tetrahedron, 65(24), 4703-4708 (2009)
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務