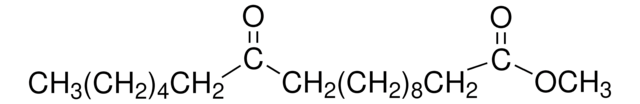
H7002
Methyl 12-hydroxystearate
≥99% (GC)
Synonym(s):
12-Hydroxystearic acid methyl ester, Methyl 12-hydroxyoctadecanoate
About This Item
Recommended Products
Assay
≥99% (GC)
form
powder
functional group
ester
lipid type
saturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCCC(O)CCCCCCCCCCC(=O)OC
InChI
1S/C19H38O3/c1-3-4-5-12-15-18(20)16-13-10-8-6-7-9-11-14-17-19(21)22-2/h18,20H,3-17H2,1-2H3
InChI key
RVWOWEQKPMPWMQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Chemical Changes of Hydroperoxy-, Epoxy-, Keto- and Hydroxy-Model Lipids under Simulated Gastric Conditions.: This study explores the stability and chemical transformations of hydroxy fatty acids, including Methyl 12-hydroxystearate, under digestive conditions, providing insight into dietary fat metabolism and its implications for nutritional sciences (Marquez-Ruiz et al., 2021).
- Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components.: This research highlights the potential environmental applications of Methyl 12-hydroxystearate, as a standard, in enhancing nitrogen cycling, important for studies on wastewater treatment and ecosystem management (Lu et al., 2014).
- Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids.: This study investigates the synthesis of derivatives of Methyl 12-hydroxystearate for potential use in food preservation and pharmaceutical applications, emphasizing its antioxidant and antifungal properties (Reddy et al., 2012).
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service