471801
4-Pentenoic anhydride
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
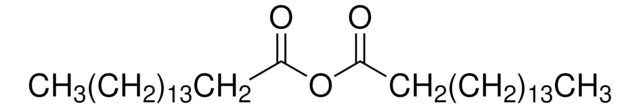
Fórmula lineal:
(H2C=CHCH2CH2CO)2O
Número de CAS:
Peso molecular:
182.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
refractive index
n20/D 1.447 (lit.)
bp
78-81 °C/0.4 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
functional group
allyl
anhydride
ester
SMILES string
C=CCCC(=O)OC(=O)CCC=C
InChI
1S/C10H14O3/c1-3-5-7-9(11)13-10(12)8-6-4-2/h3-4H,1-2,5-8H2
InChI key
NEDHQDYBHYNBIF-UHFFFAOYSA-N
Categorías relacionadas
General description
4-Pentenoic anhydride is a carboxylic anhydride.
Application
4-Pentenoic anhydride may be used:
- In the preparation of glucose functionalized (co)polymers.
- As a monomer in the preparation of cross-linked polyanhydrides.
- For the preparation of polymers with pendant vinyl or acetylene.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid.
Corredor C, et al.
Proceedings of the National Academy of Sciences of the USA, 58(6), 2299-2299 (1967)
2-(Trimethylsilyl) ethyl Glycosides. Transformation into the Corresponding 1-O-Acyl Sugars.
Ellervik U and Magnusson G.
Acta Chemica Scandinavica, 47, 826-826 (1993)
Gaojian Chen et al.
Chemical communications (Cambridge, England), (10)(10), 1198-1200 (2009-02-26)
Homopolymer and block copolymer bearing carbohydrate side chain functionality were obtained by grafting glucothiose onto alkene functional scaffolds via a thiol-ene click reaction and the resulting copolymer was used to form thermo-responsive micelles as a potential drug carrier.
Marlène Rippe et al.
Biomaterials science, 7(7), 2850-2860 (2019-05-10)
Glycosaminoglycans (GAGs) are important components of the extracellular matrix that have attracted great interest for drug delivery and pharmaceutical applications due to their diverse biological functions. Among GAGs, heparosan (Hep), a biosynthetic precursor of heparin, has recently emerged as a
Vien T Huynh et al.
Biomacromolecules, 12(5), 1738-1751 (2011-04-12)
Statistical and block copolymers based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly[oligo(ethylene glycol) methylether methacrylate] (POEGMEMA) were modified with 4-pentenoic anhydride or 4-oxo-4-(prop-2-ynyloxy)butanoic anhydride to generate polymers with pendant vinyl or acetylene, respectively. Subsequent thiol-ene or thiol-yne reaction with thioglycolic acid
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico