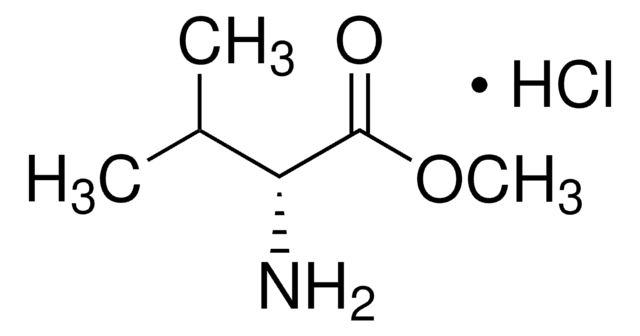
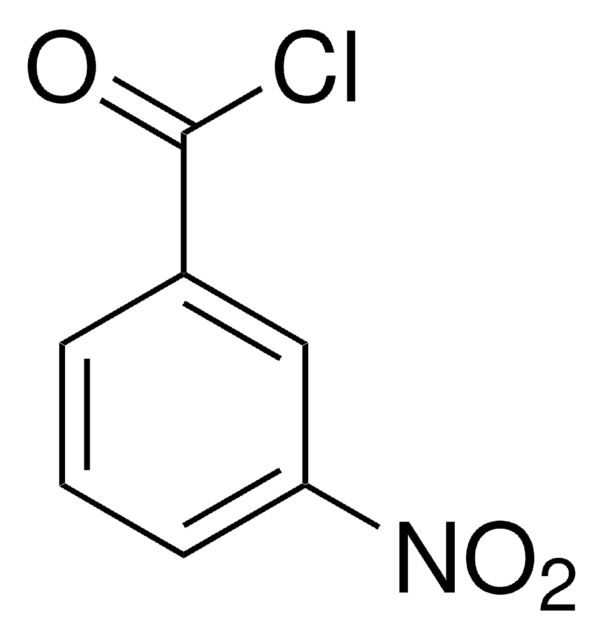
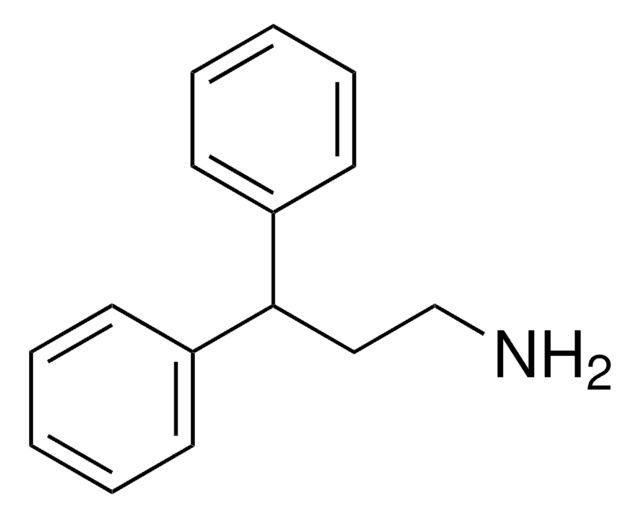
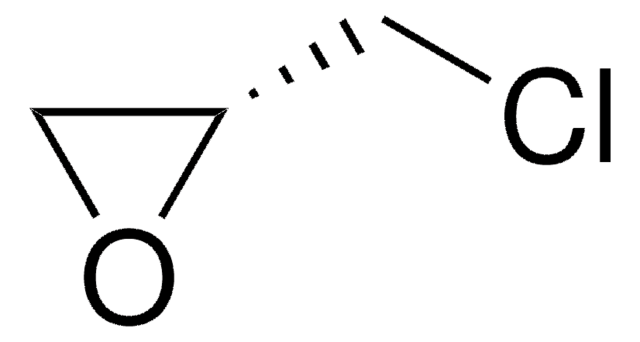
The regeneration procedure for ChiraldexTA columns is located in Supelco brochure, T407123 (JCH), A Guide to Using Cyclodextrin Bonded Phases for Chiral Separations by Capillary Gas Chromatography, on pages 8 and 9. These pages are attached.
$2,600.00
推荐产品
物料
fused silica
描述
GC capillary column
包装
pkg of 1 ea
参数
-10-180 °C temperature (isothermal or programmed)
β值
500
df
0.12 μm
技术
gas chromatography (GC): suitable
长度 × 内径
20 m × 0.25 mm
基质活性基团
non-bonded; 2,6-di-O-pentyl-3-trifluoroacetyl derivative of γ-cyclodextrin phase
应用
agriculture
chemicals and industrial polymers
cleaning products
clinical
cosmetics
environmental
flavors and fragrances
food and beverages
forensics and toxicology
life science and biopharma
personal care
pharmaceutical (small molecule)
色谱柱类型
capillary chiral
分离技术
chiral
正在寻找类似产品? 访问 产品对比指南
一般描述
化学/物理抗性
- -10 °C 至 180 °C 等温和程序的
其他说明
法律信息
商品
Chromatographic enantiomeric separation of amino acids, like proline, is described for chiral GC analysis after derivatization.
相关内容
Amino acids are building blocks of peptides and proteins. Enantiomeric separation of these molecules can be performed through chromatography by chiral stationary phases. This article describes the chiral GC analysis of one amino acid, proline, after achiral derivatization.
In this study we demonstrate the separation of D- and L-carvone enantiomers in samples of caraway seed, dill seed, native spearmint and scotch spearmint essential oils using an Astec CHIRALDEX G-TA capillary GC column.
-
What is the regeneration procedure for Chiraldex TA columns?
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门


![5-Boc-1,4,6,7-tetrahydro-pyrazolo[4,3-c]pyridine-3-carboxylic acid](/deepweb/assets/sigmaaldrich/product/structures/145/700/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f/640/2ac352d1-30ff-42c1-b2f3-0fe11cecf36f.png)