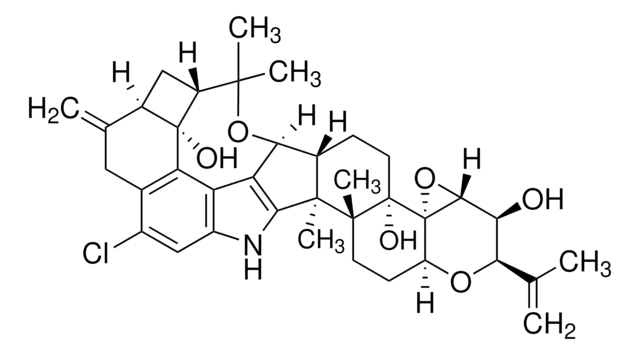
SML0406
Roquefortine C
≥98% (HPLC), from Penicillium roqueforti
别名:
2H-Pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(3H,5aH)-dione, 10b-(1,1-dimethyl-2-propen-1-yl)-6,10b,11,11a-tetrahydro-3-(1H-imidazol-5-ylmethylene)-,(3E,5aS,10bR,11aS)-, Roquefortine from Penicillium roqueforti
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
生物源
Penicillium roqueforti
品質等級
化驗
≥98% (HPLC)
溶解度
chloroform: 1 mg/mL
ethyl acetate: 1 mg/mL
DMSO: 10 mg/mL
methanol: 10 mg/mL
儲存溫度
−20°C
InChI
1S/C22H23N5O2/c1-4-21(2,3)22-10-17-18(28)25-16(9-13-11-23-12-24-13)19(29)27(17)20(22)26-15-8-6-5-7-14(15)22/h4-9,11-12,17,20,26H,1,10H2,2-3H3,(H,23,24)(H,25,28)/b16-9-
InChI 密鑰
SPWSUFUPTSJWNG-SXGWCWSVSA-N
應用
Roquefortine C has been used as a standard for the quantification of roquefortine C by high-performance liquid chromatography (HPLC). It has also been used as a standard for the quantification of roquefortine C by liquid chromatography-mass spectrometry (LC−MS/MS).
生化/生理作用
Roquefortine C is a paralytic neurotoxin of a dioxopiperazine structure produced by a diverse range of fungi, most notably Penicillium species. It has been found in blue cheese and in many other food products due to natural occurrence and contamination. Roquefortine C was found to be active on a wide range of organisms. It inhibits the growth of Gram-positive bacteria, and cockerels treated with roquefortine lost their righting reflex and died within 8-12 hours. Mice injected with roquefortine C experienced neurotoxic properties. Roquefortine C was also reported to inhibit cytochrome P450 as well as tubulin polymerization.
準備報告
Soluble in methanol (10 mg/mL), DMSO (10 mg/mL), ethyl acetate (1 mg/mL) and chloroform (1 mg/mL). DMSO solution at 10 mg/mL is stable for 3 months at −20 °C.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A natural short pathway synthesizes roquefortine C but not meleagrin in three different Penicillium roqueforti strains
Kosalkova K, et al.
Applied Microbiology and Biotechnology, 99(18), 7601-7612 (2015)
Katarina Kosalková et al.
Biochimie, 91(2), 214-225 (2008-10-28)
The biosynthesis of the beta-lactam antibiotic penicillin is an excellent model for the study of secondary metabolites produced by filamentous fungi due to the good background knowledge on the biochemistry and molecular genetics of the beta-lactam producing microorganisms. The three
David J Richard et al.
Proceedings of the National Academy of Sciences of the United States of America, 101(33), 11971-11976 (2004-05-14)
The syntheses of isoroquefortine C and a related heterocycle were achieved by implementation of both intra- and intermolecular vinyl amidation reactions. These accomplishments represent a significant advance in the use of these strategies in the generation of complex molecules.
Ramón O García-Rico et al.
International microbiology : the official journal of the Spanish Society for Microbiology, 12(2), 123-129 (2009-09-29)
Heterotrimeric G protein signaling regulates many processes in fungi, such as development, pathogenicity, and secondary metabolite biosynthesis. For example, the Galpha subunit Pga1 from Penicillium chrysogenum regulates conidiation and secondary metabolite production in this fungus. The dominant activating allele, pga1G42R
Ramón O García-Rico et al.
Microbiology (Reading, England), 154(Pt 11), 3567-3578 (2008-10-30)
We have studied the role of the pga1 gene of Penicillium chrysogenum, encoding the alpha subunit of a heterotrimeric G protein, in secondary metabolite production. The dominant activating pga1(G42R) mutation caused an increase in the production of the three secondary
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门