推荐产品
等級
pharmaceutical primary standard
API 家族
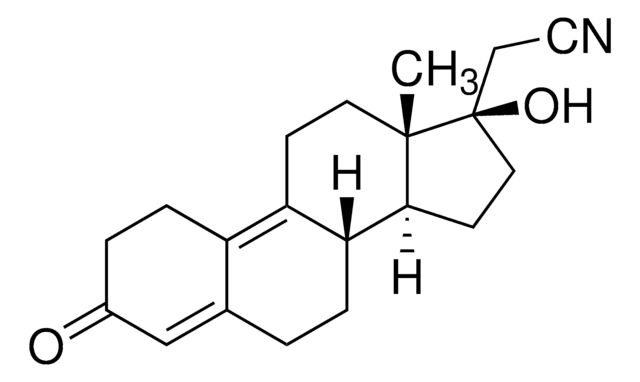
gestodene
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
CC[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1C=C[C@@]2(O)C#C
InChI
1S/C21H26O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,10,12-13,16-19,23H,3,5-9,11H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
InChI 密鑰
SIGSPDASOTUPFS-XUDSTZEESA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Gestodene EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Gestodene is a synthetic progestin used as a contraceptive. Gestodene displays a high binding affinity to the progesterone receptor, and also binds strongly to adrogen and glucocorticoid receptors.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Repr. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Pasquale Florio et al.
Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 27(6), 434-438 (2011-01-06)
In a retrospective case-control study, we compared the effectiveness of hysteroscopic correction and hormonal treatment to improve symptoms [postmestrual abnormal uterine bleeding (PAUB), pelvic pain localized in suprapubic site] associated with isthmocele. Women (n = 39; mean age ± SD
Ana Cristina Rebelo et al.
The European journal of contraception & reproductive health care : the official journal of the European Society of Contraception, 16(4), 289-297 (2011-07-22)
To evaluate the influence of oral contraceptives (OCs) containing 20 μg ethinylestradiol (EE) and 150 μg gestodene (GEST) on the autonomic modulation of heart rate (HR) in women. One-hundred and fifty-five women aged 24 ± 2 years were divided into
Sille Vaiksaar et al.
Perceptual and motor skills, 113(3), 764-772 (2012-03-13)
Eight female rowers (M age = 21.0 yr., SD = 2.8), using a monophasic oral-contraceptive pill, performed a 1-hr. rowing ergometer test (intensity: 70% VO2max) during the active-pill and non active-pill phases of the oral contraceptive cycle. No significant differences
Ana Cristina S Rebelo et al.
Contraception, 81(4), 309-315 (2010-03-17)
The purpose of this study was to evaluate the effect of long-term use of oral contraceptives (OC) containing 0.20 mg of ethinylestradiol (EE) combined with 0.15 mg of gestodene (GEST) on the peak aerobic capacity and at the anaerobic threshold
Lothar A J Heinemann et al.
Contraception, 81(5), 401-407 (2010-04-20)
This study investigated whether gestodene-containing oral contraceptives (OCs) carry a higher risk of venous thromboembolism (VTE) than OCs containing progestins other than desogestrel and gestodene. The study was conducted based on the hypothesis that the biases and confounding factors that
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门