所有图片(1)
About This Item
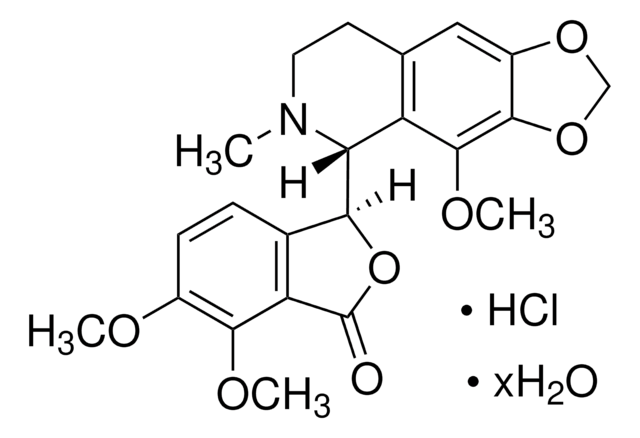
经验公式(希尔记法):
C20H24ClNO · HCl
CAS号:
分子量:
366.32
EC號碼:
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
推荐产品
等級
analytical standard
品質等級
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
forensics and toxicology
pharmaceutical (small molecule)
veterinary
格式
neat
SMILES 字串
Cl.Clc1ccc(cc1)C(OCCN2CCCCC2)c3ccccc3
InChI
1S/C20H24ClNO.ClH/c21-19-11-9-18(10-12-19)20(17-7-3-1-4-8-17)23-16-15-22-13-5-2-6-14-22;/h1,3-4,7-12,20H,2,5-6,13-16H2;1H
InChI 密鑰
UNPLRYRWJLTVAE-UHFFFAOYSA-N
應用
Cloperastine hydrochloride may be used as an analytical standard for the quantification of cloperastine in biological samples using liquid chromatography coupled to tandem mass spectrometry.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
[Clinical study of a new antitussive: cloperastine].
D Olivieri et al.
Archivio Monaldi per la tisiologia e le malattie dell'apparato respiratorio, 38(5-6), 209-218 (1983-09-01)
Gen Yamamoto et al.
Canadian journal of physiology and pharmacology, 87(11), 893-899 (2009-11-26)
We investigated the effects of the centrally acting antitussives dextromethorphan and cloperastine on urinary bladder dysfunction 24 h after cerebral infarction in rats using the cystometry technique. First, cystometrography was performed in conscious male Sprague-Dawley rats. Cerebral infarction was then
Antonia García et al.
Journal of pharmaceutical and biomedical analysis, 61, 230-236 (2012-01-10)
The classification of an impurity of a drug substance as genotoxic means that the "threshold of toxicological concern" (TTC) value of 1.5 μg/day intake, considered to be associated with an acceptable risk, should be the admissible limit in the raw
[Effect of cloperastine on muco-ciliary clearance].
D Olivieri et al.
Archivio Monaldi per la tisiologia e le malattie dell'apparato respiratorio, 38(5-6), 219-223 (1983-09-01)
Yukoh Sakata et al.
International journal of pharmaceutics, 335(1-2), 12-19 (2006-11-23)
Tablets containing both paracetamol (PM) and cloperastine hydrochloride (CLH) in a combination formulation prepared by standard vertical granulation technology were found to have altered pharmaceutical properties. The hardness and disintegration time of tablets containing both PM and CLH gradually increased
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门