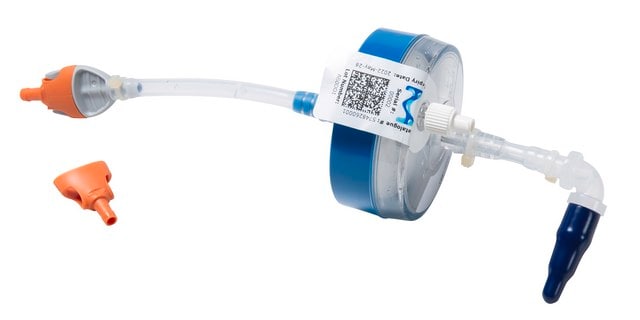
推荐产品
材料
polysulfone device
polysulfone support
品質等級
agency
meets EP 2.6.14
meets JP 4.01
meets USP 85
無菌
irradiated
sterile; γ-irradiated
產品線
Millipak® Final Fill 60
特點
hydrophilic
製造商/商標名
Millipak®
參數
10 psi max. differential pressure (0.7 bar) at 25 °C (Reverse)
4.5 L/min flow rate at 0.69 bar (ΔP)
5.5 bar max. inlet pressure (80 psi) at 25 °C
60 L process volume
80 psi max. differential pressure (5.5 bar) at 25 °C (Forward)
技術
prefiltration: suitable
過濾器過濾面積
300 cm2
雜質
<0.25 EU/mL USP bacterial endotoxins (LAL test, per device; aqueous extraction)
<0.25 EU/mL bacterial endotoxins (LAL test, per device; aqueous extraction)
基質
Durapore®
孔徑
5.0 μm pore size
起泡點
≥2 psi (138 mbar), air with water
接頭
(38 mm (1 1/2 in.) Sanitary Flange Inlet and Outlet)
正在寻找类似产品? 访问 产品对比指南
一般說明
包裝
準備報告
This product was manufactured with a Durapore® membrane which meets the criteria for a "non-fiber releasing" filter as defined in 21 CFR 210.3 (b)(6), validated based on large volume parenteral specifications as detailed in USP 788 Particulate Matter in Injections
其他說明
- Organism Retention: Microorganism
- Mode of Action: Filtration (size exclusion)
- Application: BioProcessing
- Intended Use: Reduction or removal of microorganism/bioburden
- Instructions for Use: Please refer general guidelines section of user guide shipped with this product
- Storage Statement: Please refer user guide shipped with this product
- Disposal Statement: Dispose of in accordance with applicable federal, state and local regulations
法律資訊
Not finding the right product?
Try our 产品选型工具.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门