推荐产品
化驗
≥95% (HPLC)
形狀
powder
儲存溫度
2-8°C
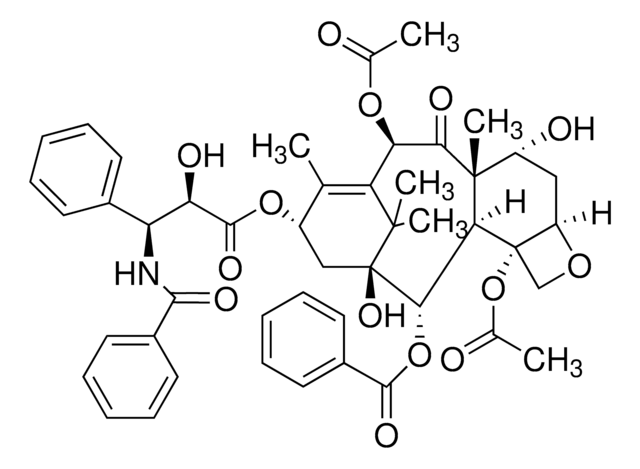
SMILES 字串
CC(=O)O[C@H]1C(=O)[C@]2(C)[C@@H](O)C[C@H]3OC[C@@]3(OC(C)=O)[C@H]2[C@H](OC(=O)c4ccccc4)[C@]5(O)C[C@H](O)C(C)=C1C5(C)C
InChI
1S/C31H38O11/c1-15-19(34)13-31(38)26(41-27(37)18-10-8-7-9-11-18)24-29(6,20(35)12-21-30(24,14-39-21)42-17(3)33)25(36)23(40-16(2)32)22(15)28(31,4)5/h7-11,19-21,23-24,26,34-35,38H,12-14H2,1-6H3/t19-,20-,21+,23+,24-,26-,29+,30-,31+/m0/s1
InChI 密鑰
OVMSOCFBDVBLFW-VHLOTGQHSA-N
正在寻找类似产品? 访问 产品对比指南
應用
紫杉酚的前体
訊號詞
Danger
危險分類
Carc. 1B - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Da Cheng Hao et al.
PloS one, 6(6), e21220-e21220 (2011-07-07)
Illumina second generation sequencing is now an efficient route for generating enormous sequence collections that represent expressed genes and quantitate expression level. Taxus is a world-wide endangered gymnosperm genus and forms an important anti-cancer medicinal resource, but the large and
Elizabeth M Heider et al.
Physical chemistry chemical physics : PCCP, 9(46), 6083-6097 (2008-01-03)
The three-dimensional structure of a unique polymorph of the anticancer drug paclitaxel (Taxol) is established using solid state NMR (SSNMR) tensor ((13)C & (15)N) and heteronuclear correlation ((1)H-(13)C) data. The polymorph has two molecules per asymmetric unit (Z' = 2)
Hiroshi Izumi et al.
The Journal of organic chemistry, 73(6), 2367-2372 (2008-02-16)
The comparison between measured and conformer-weighted calculated VCD spectra of the baccatin III ring of paclitaxel and visualization of the conformations using the new code for structure-activity relationships are reported for the first time. The VCD spectrum of paclitaxel closely
Mark E Ondari et al.
The Journal of organic chemistry, 74(5), 2186-2188 (2009-02-10)
A one-pot trisilylation step to protect three hydroxyl groups of baccatin III (1), followed by hydride ester cleavage and base hydrolysis of a triethylsilyl ether at C13, provides efficient access to a key intermediate 9 (top path). This route removes
Catherine Loncaric et al.
Chemistry & biology, 13(3), 309-317 (2006-04-28)
The 10beta-acetyltransferase on the biosynthetic pathway of the antineoplastic drug Taxol catalyzes the regiospecific transfer of the acetyl group of acetyl-coenzyme A (CoA) to 10-deacetylbaccatin III. We demonstrate that in addition to acetyl group transfer, the overexpressed enzyme also catalyzes
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门