所有图片(1)
选择尺寸
变更视图
500 MG
$177.00
2.5 G
$592.00
About This Item
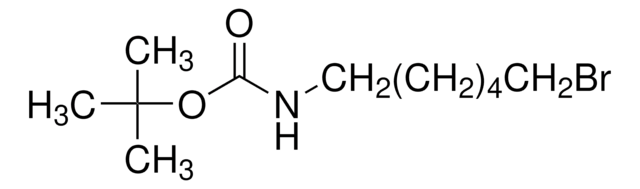
线性分子式:
Br(CH2)4NHCO2C(CH3)3
CAS号:
分子量:
252.15
MDL编号:
UNSPSC代码:
12352200
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
等级
technical
质量水平
方案
≥90% (AT)
表单
liquid
反应适用性
reagent type: cross-linking reagent
杂质
~5% 1-boc-pyrrolidine
官能团
Boc
amine
bromo
储存温度
2-8°C
SMILES字符串
BrCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H18BrNO2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7H2,1-3H3,(H,11,12)
InChI key
GKGFAEREWWZBKY-UHFFFAOYSA-N
应用
4-(Boc-amino)butyl bromide can be used:
- For the synthesis of N-Boc-aminoalkoxyphenyl derivatives, precursor to pharmacophore elements for the treatment of glaucoma.[1]
- For the synthesis of various aloperine derivatives with potential application as anti-HIV agents.[2]
- For the modification of 4,5,6,7-tetrabromobenzotriazole (TBB) derivatives to generate improved CK2 inhibitors.[3]
警示用语:
Danger
危险声明
危险分类
Eye Dam. 1
储存分类代码
10 - Combustible liquids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Identification of bivalent ligands with melatonin receptor agonist and fatty acid amide hydrolase (FAAH) inhibitory activity that exhibit ocular hypotensive effect in the rabbit.
Spadoni G, et al.
Journal of medicinal chemistry, 61(17), 7902-7916 (2018)
Structure Optimization of Aloperine Derivatives as HIV-1 Entry Inhibitors.
Dang Z, et al.
ACS Medicinal Chemistry Letters, 8(11), 1199-1203 (2017)
Synthesis, biological activity and structural study of new benzotriazole-based protein kinase CK2 inhibitors.
Swider R, et al.
Royal Society of Chemistry Advances, 5(89), 72482-72494 (2015)
H. Ina et al.
The Journal of Organic Chemistry, 61, 1023 -1023 (1995)
D.L. Selwood et al.
Journal of Medicinal Chemistry, 4, 78-78 (2001)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持