推荐产品
表单
powder
mp
138-143 °C
官能团
amine
hydroxyl
phenyl
SMILES字符串
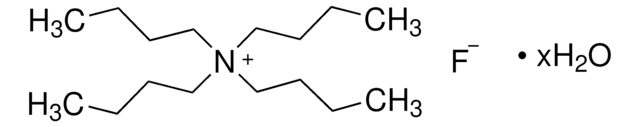
CCCC[N+](CCCC)(CCCC)CCCC.OC1(C2=CC=CC=C2)C3=C(C=CC=C3)C4=C1C=CC=C4.OC5(C6=CC=CC=C6)C7=C(C=CC=C7)C8=C5C=CC=C8.OC9(C%10=CC=CC=C%10)C%11=C(C=CC=C%11)C%12=C9C=CC=C%12.[F-]
InChI
1S/3C19H14O.C16H36N.FH/c3*20-19(14-8-2-1-3-9-14)17-12-6-4-10-15(17)16-11-5-7-13-18(16)19;1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h3*1-13,20H;5-16H2,1-4H3;1H/q;;;+1;/p-1
InChI key
HTKORNSKGASQCX-UHFFFAOYSA-M
一般描述
TBAF(9-Ph-9-fluorenol)3 is a fluoride–alcohol complex. It is formed by reacting 9-phenylfluoren-9-ol with tetrabutylammonium fluoride trihydrate (TBAF(H2O)3). The geometric structure and bonding of TBAF(9-Ph-9-fluorenol)3 have been investigated.[1]
应用
Nucleophilic fluorinating reagent is a more convenient crystalline solid for handling and storage compared to tetrabutylammonium fluoride hydrate (Aldrich 241512). As reported by Engle and Gouverneur, these solids display comparable reactivity on similar substrates.
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Coordination diversity in hydrogen-bonded homoleptic fluoride?alcohol complexes modulates reactivity.
Engle KM, et al.
Chemical Science, 6(9), 5293-5302 (2015)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门