所有图片(1)
选择尺寸
变更视图
10 G
$134.00
50 G
$445.00
About This Item
线性分子式:
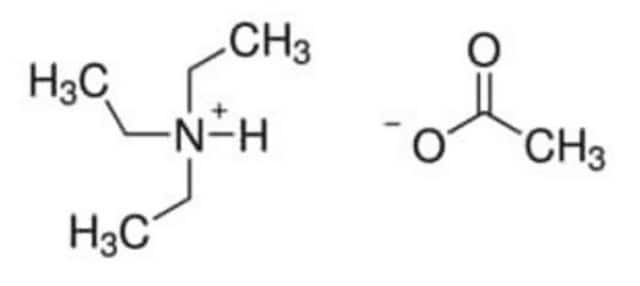
(CH3CH2CH2CH2)4N(OCOCH3)
CAS号:
分子量:
301.51
Beilstein:
3599376
EC 号:
MDL编号:
UNSPSC代码:
12352116
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
等级
technical
质量水平
方案
≥90% (T)
表单
powder
mp
95-98 °C (lit.)
SMILES字符串
CC([O-])=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.C2H4O2/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;1-2(3)4/h5-16H2,1-4H3;1H3,(H,3,4)/q+1;/p-1
InChI key
MCZDHTKJGDCTAE-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
一般描述
Tetrabutylammonium acetate is an effective alternative for sodium acetate (NaOAc) due to its good solubility in organic solvents.[1]
应用
Tetrabutylammonium acetate (TBAAc) is a good source of nucleophilic acetate ion for SN2 substitution reactions. It is commonly used to displace sulfonates and allylic halides to get corresponding acetates.[1][2] Additionally, TBAAc can also be used as a mild, soluble base in Sonogashira reaction[3] and Heck arylation.[4]
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Ligand-, copper-, and amine-free Sonogashira reaction of aryl iodides and bromides with terminal alkynes.
Urgaonkar S and Verkade JG
The Journal of Organic Chemistry, 69(17), 5752-5755 (2004)
Tetrabutylammonium Acetate.
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2001)
Secondary deuterium kinetic isotope effects and the intervention of nonclassical ions in the solvolysis of exo-norborn-2-yl bromobenzene-p-sulfonate.
Maskill H,
Journal of the American Chemical Society, 98(26), 8482-8485 (1976)
T. Jeffery, M. David
Tetrahedron Letters, 39, 5751-5751 (1998)
Pd nanoparticle catalyzed Heck arylation of 1, 1-disubstituted alkenes in ionic liquids. Study on factors affecting the regioselectivity of the coupling process.
Calo V, et al.
Organometallics, 22(21), 4193-4197 (2003)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门