682365
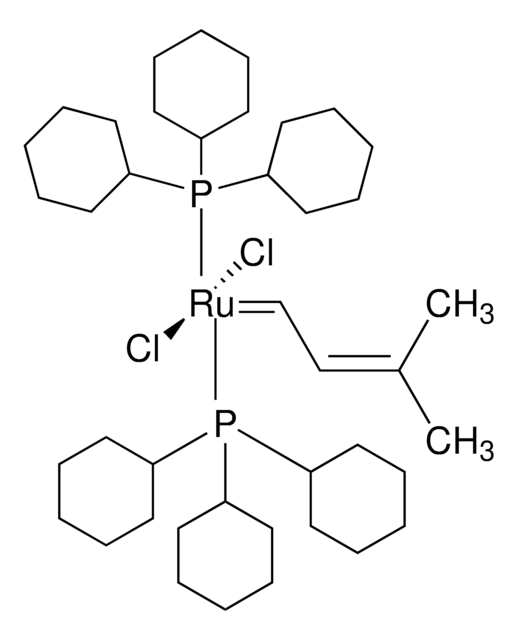
Grubbs催化剂® M207
Umicore
别名:
二氯[1,3-双(2,4,6-三甲基苯基)-2-咪唑烷亚基](3-甲基-2-亚丁烯基)(三环己基膦)钌(II), Grubbs催化剂® M2b (C827), [SIMes]二氯(3-甲基-2-亚丁烯基)(三环己基膦)Ru(II), 异亚戊烯基(1,3-双(2,4,6-三甲基苯基)-咪唑烷-2-亚基)(三环己基膦)二氯化钌(II)
About This Item
推荐产品
反應適用性
core: ruthenium
reagent type: catalyst
reaction type: Olefin Metathesis
儲存溫度
2-8°C
SMILES 字串
C1CCC(CC1)P(C2CCCCC2)C3CCCCC3.C\C(C)=C/C=[Ru](Cl)(Cl)=C4N(CCN4c5c(C)cc(C)cc5C)c6c(C)cc(C)cc6C
InChI
1S/C21H26N2.C18H33P.C5H8.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-4-5(2)3;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1,4H,2-3H3;2*1H;/q;;;;;+2/p-2
InChI 密鑰
LCOFYVWULBZOTA-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
應用
法律資訊
产品许可证
该产品及其制造或使用受Umicore PMC拥有或控制的一项或多项已发布或正在申请的美国专利(和国外等同专利)权限管辖。通过Sigma-Aldrich、其关联公司或其授权分销商从Umicore PMC购买此产品,购买者将享受有限的一次性、非排他、不可转让、不可分配的许可。购买者使用此产品可能会侵犯第三方拥有或控制的专利。购买者应自行确保其使用产品未侵犯第三方专利权或超出本协议授予的许可范围。
有关产品的更多信息,请询当地Umicore PMC联系人,网址http://www.pmc.umicore.com。
訊號詞
Warning
危險聲明
危險分類
Flam. Sol. 2
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
相关内容
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



双(3-溴吡啶)钌(II)](/deepweb/assets/sigmaaldrich/product/structures/261/898/e64eea4e-5a09-4c7d-b400-c43b3517de2a/640/e64eea4e-5a09-4c7d-b400-c43b3517de2a.png)

![二氯[1,3-双(2,4,6-三甲基苯基)-2-咪唑烷亚基][3-(2-吡啶基)亚丙基]钌(II)](/deepweb/assets/sigmaaldrich/product/structures/416/586/0907c050-f5d7-4a53-b22f-23170c2703c2/640/0907c050-f5d7-4a53-b22f-23170c2703c2.png)