推荐产品
品質等級
化驗
97%
mp
61-65 °C (lit.)
官能基
phenyl
SMILES 字串
C1CC(CCN1)c2ccccc2
InChI
1S/C11H15N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-5,11-12H,6-9H2
InChI 密鑰
UTBULQCHEUWJNV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Clandestine drug synthesis.
W H Soine
Medicinal research reviews, 6(1), 41-74 (1986-01-01)
Barbara Wenzel et al.
Bioorganic & medicinal chemistry letters, 22(6), 2163-2166 (2012-03-01)
This Letter describes the synthesis of two regioisomers of a new class of vesamicol analogs as possible ligands for imaging the vesicular acetylcholine transporter in future PET studies. The two pyrrolovesamicols (±)-6a and (±)-6b were synthesized by nucleophilic ring opening
A G Ishkov et al.
Voprosy meditsinskoi khimii, 38(2), 25-28 (1992-03-01)
A rate of utilization of 4-phenyl piperidine and its 12 derivatives by brain monoamine oxidase (MAO) was studied as compared with typical neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The enzyme was isolated from P2 synaptosomal fraction of brain corpus striatum of Sprague-Dawley rats.
M G Russell et al.
Journal of medicinal chemistry, 35(11), 2025-2033 (1992-05-29)
This paper describes the synthesis of some conformationally restricted 4-phenylpiperidine analogues and their affinities for the guinea pig cerebellum sigma recognition site ([3H]-DTG) and the rat striatum dopamine D2 receptor ([3H]-(-)-sulpiride) in order to develop potent selective sigma ligands as
Diane K Luci et al.
Bioorganic & medicinal chemistry letters, 17(23), 6489-6492 (2007-10-16)
Various 4-phenylpiperidine-benzoxazin-3-ones were synthesized and biologically evaluated as urotensin-II (U-II) receptor antagonists. Compound 12i was identified from in vitro evaluation as a low nanomolar antagonist against both rat and human U-II receptors. This compound showed in vivo efficacy in reversing
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门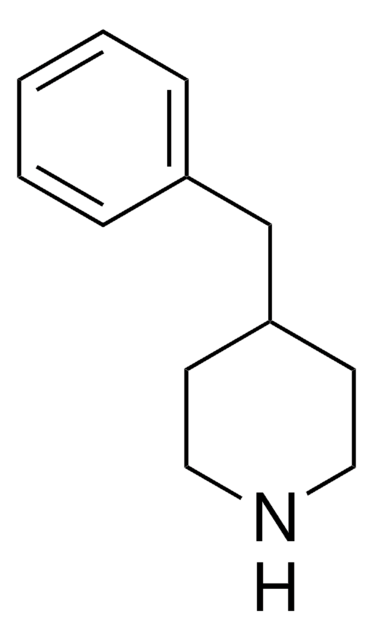





![苯并[b]噻吩-2-基硼酸 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)

![9-氮杂双环[3.3.1]壬烷N-氧基 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)

