565814
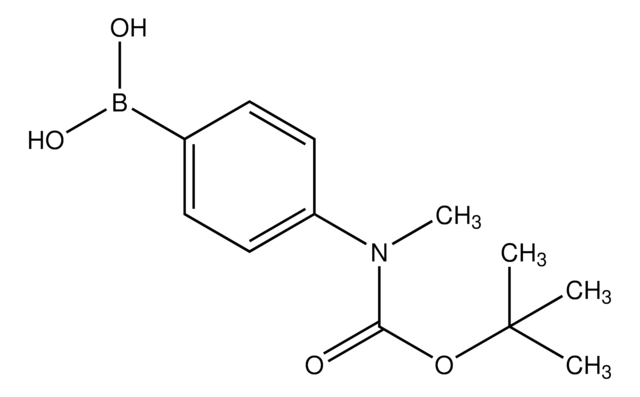
4-(N-Boc-氨基)苯硼酸
≥95.0%
别名:
4-(Boc-amino)phenylboronic acid, 4-[(tert-Butoxycarbonyl)amino]benzeneboronic acid, C-(1,1-Dimethylethyl) N-(4-boronophenyl)carbamate, [4-(N-Bocamino)phenyl]boronic acid, [4-(tert-Butoxycarbonylamino)phenyl]boronic acid, [4-[[[(1,1-Dimethylethyl)oxy]carbonyl]amino]phenyl]boronic acid
登录查看公司和协议定价
所有图片(2)
About This Item
线性分子式:
(CH3)3CO2CNHC6H4B(OH)2
CAS号:
分子量:
237.06
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥95.0%
形狀
solid
mp
199-204 °C (dec.) (lit.)
SMILES 字串
CC(C)(C)OC(=O)Nc1ccc(cc1)B(O)O
InChI
1S/C11H16BNO4/c1-11(2,3)17-10(14)13-9-6-4-8(5-7-9)12(15)16/h4-7,15-16H,1-3H3,(H,13,14)
InChI 密鑰
UBVOLHQIEQVXGM-UHFFFAOYSA-N
應用
用于研究内消旋环烯丙基二碳酸酯通过 SN2′ 取代的铑催化去对称作用的硼酸。
其他說明
含有不定量的酸酐
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Frederic Menard et al.
Organic letters, 8(20), 4569-4572 (2006-09-22)
An enantio-, regio-, and diastereoselective rhodium(I)-catalyzed desymmetrization of a meso-cyclic allylic dicarbonate with organoboronic acid nucleophiles is described. The rhodium(I) catalyst formed in situ from [Rh(cod)OH]2 and Xyl-P-PHOS allowed the S(N)2' allylic substitution product to be obtained with a range
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门