所有图片(1)
选择尺寸
变更视图
5 G
$275.00
25 G
$884.00
About This Item
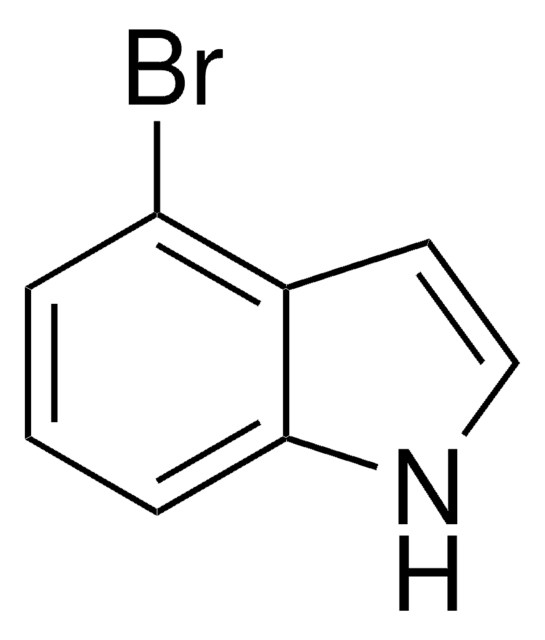
经验公式(希尔记法):
C13H14BrNO2
CAS号:
分子量:
296.16
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
97%
表单
solid
mp
56-57 °C (lit.)
官能团
bromo
SMILES字符串
CC(C)(C)OC(=O)n1ccc2cc(Br)ccc12
InChI
1S/C13H14BrNO2/c1-13(2,3)17-12(16)15-7-6-9-8-10(14)4-5-11(9)15/h4-8H,1-3H3
InChI key
PBWDRTGTQIXVBR-UHFFFAOYSA-N
一般描述
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Sylvain Petit et al.
ChemMedChem, 4(2), 261-275 (2008-12-05)
The lead compound 5-bromoindolyl-3-acetohydroxamic acid (10) was recently identified as a potent inhibitor of bacterial peptide deformylases (PDFs). The synthesis and associated activities of new variants were investigated at position 5 to optimize the fit at the S1' subsite and
Hiroyuki Nakamura et al.
The Journal of organic chemistry, 70(6), 2357-2360 (2005-03-12)
[reaction: see text] Propargylic diisopropylamines containing heterocycles, which were prepared readily from heterocyclic bromides and propargyldiisopropylamine by the Sonogashira coupling reaction, underwent the allene transformation reaction in the presence of Pd(2)(dba)(3).CHCl(3) catalyst (2.5 mol %) and 1,2-bis[bis(pentafluorophenyl)phosphino]ethane (10 mol %)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持