所有图片(3)
About This Item
经验公式(希尔记法):
C7H7Br2N
CAS号:
分子量:
264.95
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
85-87 °C (lit.)
SMILES 字串
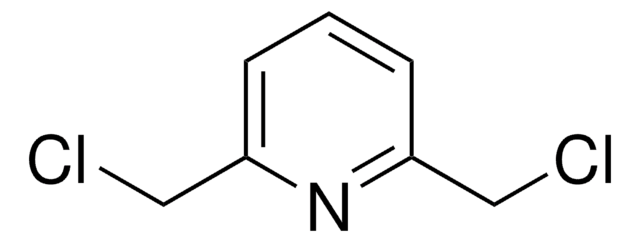
BrCc1cccc(CBr)n1
InChI
1S/C7H7Br2N/c8-4-6-2-1-3-7(5-9)10-6/h1-3H,4-5H2
InChI 密鑰
QUTSYCOAZVHGGT-UHFFFAOYSA-N
一般說明
2,6-二(溴甲基)吡啶晶体具有通过 c 滑移面作用相关的分子。分子沿 c 轴排列成堆。其参与双阳离子咪唑连接环番的合成。
應用
2,6-双(溴甲基)吡啶可用于制备以下物质:
- 一种新的吡啶-吡唑衍生物,2,6-双(3,5-二苯吡唑-1-基甲基)吡啶
- 一个大的大环配体,N(1),N(7)-吡啶-2,6-二甲基-N(2),N(6)-双((6-(3-(1H苯并[d] 咪唑-1-基)丙酰胺基)吡啶-2-基)吡啶-2,6-二甲酰胺二溴
- 小环、潜在三齿 Se (2) N (吡啶基)-供体大环
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Hanna S Abbo et al.
Molecules (Basel, Switzerland), 18(4), 4728-4738 (2013-04-24)
The tridentate ligand 2,6-bis(3,5-diphenylpyrazol-1-ylmethyl)pyridine, abbreviated as 2,6-[(3,5-ph₂pz-CH₂)₂-py], a new pyridine-pyrazole derivative, was prepared from 2,6-bis(bromomethyl)pyridine and 3,5-diphenylpyrazole. The ligand was characterized by means of elemental analyses, ATR-IR, ¹H- and ¹³C-NMR spectroscopy and single crystal X-ray crystallography. Using this ligand, a
Kim Meyer et al.
Dalton transactions (Cambridge, England : 2003), 41(46), 14059-14067 (2012-09-11)
Treatment of N(2),N(6)-bis(6-acrylamidopyridin-2-yl)pyridine-2,6-dicarboxamide with benzimidazole gives the acyclic aza-Michael addition product N(2),N(6)-bis(6-(3-(1H-benzo[d]imidazol-1-yl)propanamido)pyridin-2-yl)pyridine-2,6-dicarboxamide (2). The macrocycle N(1),N(7)-pyridine-2,6-dimethyl-N(2),N(6)-bis(6-(3-(1H-benzo[d]imidazol-1-yl)propanamido)pyridin-2-yl)pyridine-2,6-dicarboxamide dibromide ([H(2)L(2)]Br(2)) is formed through the double alkylation of 2 with 2,6-bis(bromomethyl)pyridine. The imidazole analogues of 2 and [H(2)L(2)]Br(2) (1 and [H(2)L(1)]Br(2), respectively) have
2, 6-Bis(bromomethyl) pyridine.
Cuzan O, et al.
Acta Crystallographica Section E, Structure Reports Online, 70(1), 4-4 (2014)
Synthesis of an imidazolium-linked cyclophane from histamine.
Durmus S, et al.
Tetrahedron, 61(1), 97-101 (2005)
William Levason et al.
Dalton transactions (Cambridge, England : 2003), (23)(23), 4569-4577 (2009-06-03)
Simultaneous dropwise addition of thf/EtOH solutions of Se{(CH(2))(3)OTs}(2) and o-C(6)H(4)(CH(2)SeCN)(2) or NCSe(CH(2))(3)SeCN to a suspension of NaBH(4) in thf/EtOH at room temperature yields gram quantities of the 13- and 12-membered triselenoether macrocycles (1) and (2) respectively in high yield. The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门