所有图片(3)
About This Item
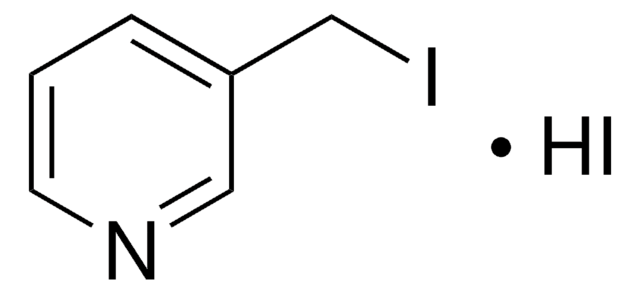
经验公式(希尔记法):
C6H6BrN · HBr
CAS号:
分子量:
252.93
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
mp
189-192 °C (lit.)
SMILES 字串
Br[H].BrCc1ccncc1
InChI
1S/C6H6BrN.BrH/c7-5-6-1-3-8-4-2-6;/h1-4H,5H2;1H
InChI 密鑰
VAJUUDUWDNCECT-UHFFFAOYSA-N
一般說明
4-(溴甲基)吡啶氢溴酸盐是取代的吡啶。它与 1,2-乙二胺和 1,3-丙二胺反应生成相应的二胺。
應用
4-(溴甲基)吡啶氢溴酸盐可用于制备:
- 3-(4-吡啶基甲基)-2′,3′-二-O-油基-5′-O-(4,4′-二甲氧基三苯基甲基)尿苷
- 3-(4-吡啶基甲基)-3′-O-油基-5′-O-(4,4-二甲氧基三苯甲基)-胸苷
- 1,4-双(N-己基-4-吡啶)丁二烯二氯酸盐
- 2-吗啉-4-基-7-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
- 8-甲基-2-吗啉-4-基-7-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
- 2-吗啉-4-基-8-(吡啶-4-基甲氧基)-4H-1,3-苯并恶嗪-4-酮
訊號詞
Danger
危險聲明
危險分類
Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Photoinduced electron transfer in supramolecular complexes of a p-extended viologen with porphyrin monomer and dimer.
Fukuzumi S, et al.
Royal Society of Chemistry Advances, 2(9), 3741-3747 (2012)
Cristiane F da Costa et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 63(1), 40-42 (2008-02-12)
We report in this work the preparation and the in vitro antileishmanial activity of a series of long chains N-monoalkylated diamines and two pyridinediamine derivatives. Several compounds, tested for their in vitro antiproliferative activity against Leishmania amazonensis and Leishmania chagasi
Luca Simeone et al.
Molecular bioSystems, 7(11), 3075-3086 (2011-09-08)
Novel thymidine- or uridine-based nucleolipids, containing one hydrophilic oligo(ethylene glycol) chain and one or two oleic acid residues (called ToThy, HoThy and DoHu), have been synthesized with the aim to develop bio-compatible nanocarriers for drug delivery and/or produce pro-drugs. Microstructural
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门