推荐产品
化驗
98%
形狀
solid
mp
128-131 °C (lit.)
SMILES 字串
CC1(C)CC(C(N)=O)C(C)(C)N1
InChI
1S/C9H18N2O/c1-8(2)5-6(7(10)12)9(3,4)11-8/h6,11H,5H2,1-4H3,(H2,10,12)
InChI 密鑰
POAGFQOGFRYOFM-UHFFFAOYSA-N
一般說明
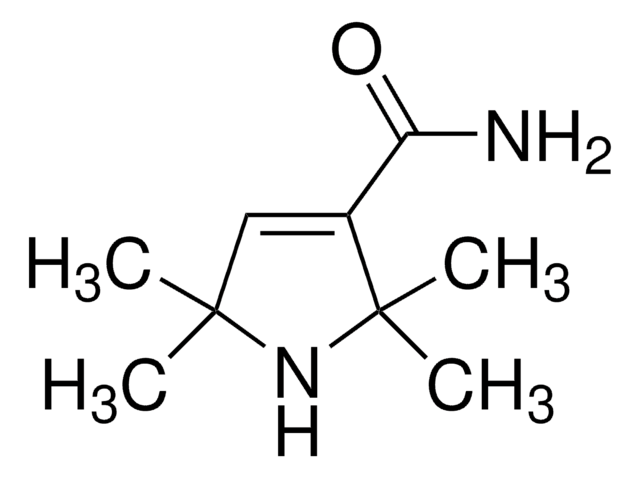
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide (2,2,5,5-Tetramethylpyrrolidine-3-carboxamide) is a carboxamide of hydrogenated pyrrole derivative. Its synthesis by the hydrogenation of 2,2,5,5-tetramethyl-3-pyrroline-3-carboxamide has been reported. The antiarrhythmic activity of some of the derivatives of 2,2,5,5-tetramethylpyrrolidine-3-carboxamide has been evaluated.
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide, a pyrrolidine derivative, is a cyclic (five-membered ring) secondary amine having four carbon atoms and one nitrogen atom. Various physical properties (freezing point, boiling point, density and refractive index) of 2,2,5,5-tetramethyl-3-pyrrolidinecarboxamidehave been reported.
應用
2,2,5,5-Tetramethyl-3-pyrrolidinecarboxamide may be used in the synthesis of its nitroxide, 3-carbamoyl-2,2,5,5-tetramethyl-1-pyrrolidinyloxy free radical.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Nitroxide free radicals in the hydrogenated pyrrole series.
Rozantsev EG, et al.
Russian Chemical Bulletin, 15(4), 638-641 (1966)
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 260-260 (2015)
O H Hankovszky et al.
Journal of medicinal chemistry, 29(7), 1138-1152 (1986-07-01)
N-(omega-Aminoalkyl)-2,2,5,5-tetramethyl-3-pyrroline- or -pyrrolidine-3-carboxamides were acylated on the primary amino group of the side chain by means of reactive acid derivatives (acid chlorides, activated esters, phthalic anhydrides, phthalimide, 2-alkyl-4H-3,1-benzoxazin-4-ones) or they were alkylated by forming the Schiff bases and subsequent sodium
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门