推荐产品
质量水平
方案
97%
表单
solid
旋光性
[α]20/D −200°, c = 1 in chloroform
mp
174-176 °C (lit.)
官能团
ester
SMILES字符串
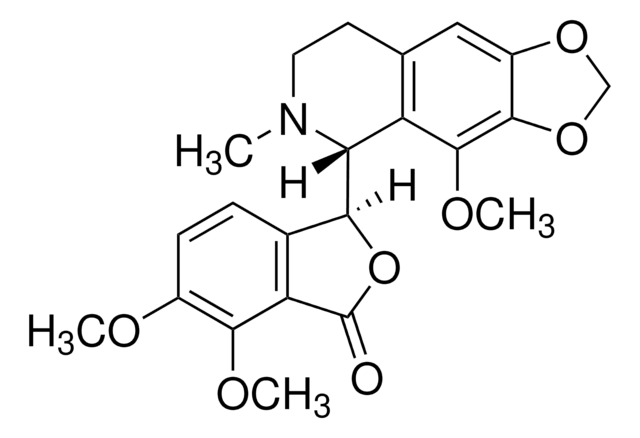
COc1ccc2C(OC(=O)c2c1OC)C3N(C)CCc4cc5OCOc5c(OC)c34
InChI
1S/C22H23NO7/c1-23-8-7-11-9-14-20(29-10-28-14)21(27-4)15(11)17(23)18-12-5-6-13(25-2)19(26-3)16(12)22(24)30-18/h5-6,9,17-18H,7-8,10H2,1-4H3/t17-,18+/m1/s1
InChI key
AKNNEGZIBPJZJG-MSOLQXFVSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
(S,R)-Noscapine is a phthalideisoquinoline alkaloid found in opium. It is an antimicrotubule agent that also shows potent antitumor activity.[1]
警示用语:
Warning
危险声明
预防措施声明
危险分类
Acute Tox. 4 Oral - STOT SE 3
靶器官
Central nervous system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Niyati Jhaveri et al.
Cancer letters, 312(2), 245-252 (2011-09-20)
Noscapine, a common oral antitussive agent, has been shown to have potent antitumor activity in a variety of cancers. Treatment of glioblastoma multiforme (GBM) with temozolomide (TMZ), its current standard of care, is problematic because the tumor generally recurs and
Cloning and characterization of canadine synthase involved in noscapine biosynthesis in opium poppy.
Thu-Thuy T Dang et al.
FEBS letters, 588(1), 198-204 (2013-12-10)
Noscapine biosynthesis in opium poppy is thought to occur via N-methylcanadine, which would be produced through 9-O-methylation of (S)-scoulerine, methylenedioxy bridge formation on (S)-tetrahydrocolumbamine, and N-methylation of (S)-canadine. Only scoulerine 9-O-methyltransferase has been functionally characterized. We report the isolation and
Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation.
Zhou J, et al.
Molecular Pharmacology, 63(4), 799-807 (2003)
Pradeep K Naik et al.
Journal of computer-aided molecular design, 26(2), 233-247 (2011-12-16)
Our screen for tubulin-binding small molecules that do not depolymerize bulk cellular microtubules, but based upon structural features of well known microtubule-depolymerizing colchicine and podophyllotoxin, revealed tubulin binding anti-cancer property of noscapine (Ye et al. in Proc Natl Acad Sci
Jitender Madan et al.
Molecular pharmaceutics, 9(5), 1470-1480 (2012-05-01)
We have previously shown that a novel microtubule-modulating noscapinoid, EM011 (9-Br-Nos), displays potent anticancer activity by inhibition of cellular proliferation and induction of apoptosis in prostate cancer cells and preclinical mice models. However, physicochemical and cellular barriers encumber the development
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持