所有图片(1)
About This Item
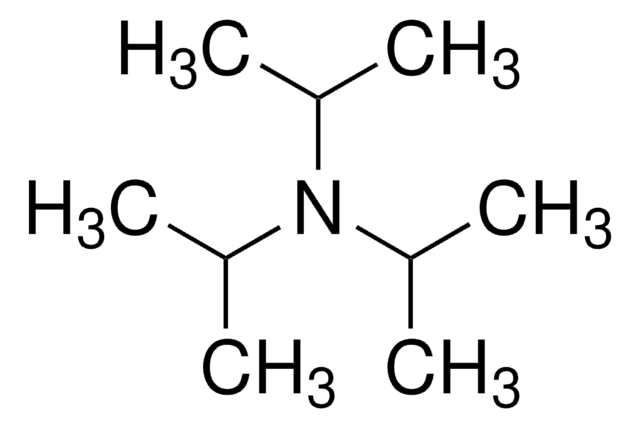
线性分子式:
(CH3)2NCH[OC(CH3)3]2
CAS号:
分子量:
203.32
Beilstein:
969629
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
推荐产品
等級
technical grade
化驗
≥90%
折射率
n20/D 1.413 (lit.)
bp
56-57 °C/8 mmHg (lit.)
密度
0.848 g/mL at 25 °C (lit.)
SMILES 字串
CN(C)C(OC(C)(C)C)OC(C)(C)C
InChI
1S/C11H25NO2/c1-10(2,3)13-9(12(7)8)14-11(4,5)6/h9H,1-8H3
InChI 密鑰
DBNQIOANXZVWIP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N,N-Dimethylformamide di-tert-butyl acetal is suitable reagent for use in the protection of carboxylic acid moiety of peroxisome proliferator-activated receptor-gamma (PPARgamma) agonist (11)C-GW7845 ((S)-2-(1-carboxy-2-{4-[2-(5-methyl-2-phenyloxazol-4-yl)ethoxy]phenyl}ethylamino)benzoic acid methyl ester). It is suitable for use in the preparation of 3-O-tert-butylmorphine. It may be used in the synthesis of tert-butylesters of pyrrole- and indolecarboxylic acids.
取代透過
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
95.0 °F - closed cup
閃點(°C)
35 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
William B Mathews et al.
Journal of nuclear medicine : official publication, Society of Nuclear Medicine, 46(10), 1719-1726 (2005-10-06)
The goal of this study was to synthesize and evaluate in vivo the peroxisome proliferator-activated receptor-gamma (PPARgamma) agonist (11)C-GW7845 ((S)-2-(1-carboxy-2-{4-[2-(5-methyl-2-phenyloxazol-4-yl)ethoxy]phenyl}ethylamino)benzoic acid methyl ester) ((11)C-compound 1). PPARgamma is a member of a family of nuclear receptors that plays a central role
E Mohacsi et al.
Journal of medicinal chemistry, 25(10), 1264-1266 (1982-10-01)
3-O-tert-Butylmorphine (5) was prepared from 6-O-acetylmorphine (3) via alkylation with N,N-dimethylformamide di-tert-butyl acetal, followed by hydrolytic removal of the 3-(dimethylamino)-2-propenoate group. The same process was used to prepare the tert-butyl ether of levorphanol (6), (-)-3-tert-butoxy-N-methylmorphinan (8). Both 5 and 8
Convenient synthesis of pyrrole-and indolecarboxylic acid tert-butylesters.
Ludwig J and Lehr M.
Synthetic Communications, 34(20), 3691-3695 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门