所有图片(2)
About This Item
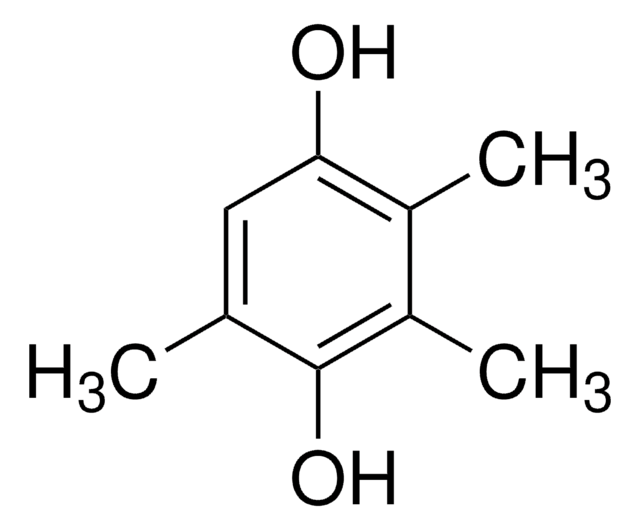
线性分子式:
(CH3)2C6H2-1,4-(OH)2
CAS号:
分子量:
138.16
Beilstein:
636976
MDL號碼:
分類程式碼代碼:
12162002
PubChem物質ID:
NACRES:
NA.23
推荐产品
化驗
97%
形狀
powder
mp
223-225 °C (lit.)
SMILES 字串
Cc1c(C)c(O)ccc1O
InChI
1S/C8H10O2/c1-5-6(2)8(10)4-3-7(5)9/h3-4,9-10H,1-2H3
InChI 密鑰
BXJGUBZTZWCMEX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,3-Dimethylhydroquinone is an alkyl p-hydroquinone that can be used as a chain breaking antioxidant and an electron donor for redox intermediates. It acts as an antioxidant due to its characteristic to terminate kinetic chains on reaction with peroxy radicals.
應用
2,3-Dimethylhydroquinone can be used as an antioxidant for lipid peroxidation. It is also used in the synthesis of benzofuran-5-ols which can further be utilized as antifungal agents in biological applications.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Substituted p-hydroquinones as inhibitors of lipid peroxidation
Roginsky V, et al.
Chemistry and Physics of Lipids, 125(1), 49-58 (2003)
Electrochemical oxidation of 2, 3-dimethylhydroquinone in the presence of 1, 3-dicarbonyl compounds
Hosseiny D, et al.
The Journal of Organic Chemistry, 71(5), 2139-2142 (2006)
Synthesis and antifungal activity of benzofuran-5-ols
Ryu C, et al.
Bioorganic & Medicinal Chemistry Letters, 20(22), 6777-6780 (2010)
Alnald Javier et al.
Dalton transactions (Cambridge, England : 2003), 43(39), 14798-14805 (2014-08-28)
Previous studies, based on thin-layer electrochemistry (TLE), in situ scanning tunneling microscopy (EC-STM), high-resolution electron energy loss spectroscopy (HREELS) and density functional theory (DFT) computations, on the chemical adsorption of hydroquinone from aqueous solutions onto atomically smooth Pd (and Pt)
Marco Persico et al.
Scientific reports, 7, 45485-45485 (2017-04-07)
In the present work we performed a combined experimental and computational study on the interaction of the natural antimalarial endoperoxide plakortin and its synthetic analogue 4a with heme. Obtained results indicate that the studied compounds produce reactive carbon radical species
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门