所有图片(1)
选择尺寸
变更视图
100 ML
$112.00
500 ML
$447.00
About This Item
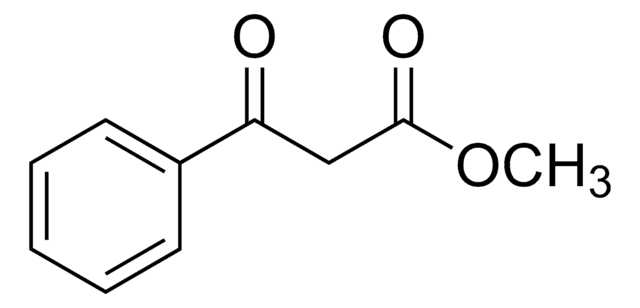
线性分子式:
C6H5COCH2COOC2H5
CAS号:
分子量:
192.21
Beilstein:
389944
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
等级
technical grade
蒸汽密度
6.6 (vs air)
方案
90%
表单
liquid
折射率
n20/D 1.52 (lit.)
沸点
265-270 °C (lit.)
溶解性
alcohol: miscible
diethyl ether: miscible
water: insoluble
密度
1.11 g/mL at 25 °C (lit.)
官能团
ester
ketone
phenyl
SMILES字符串
CCOC(=O)CC(=O)c1ccccc1
InChI
1S/C11H12O3/c1-2-14-11(13)8-10(12)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3
InChI key
GKKZMYDNDDMXSE-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
Ethyl benzoylacetate is an ester. It undergoes microbial reduction by bakers′ yeast (Saccharomyces cerevisiae), Beauveria sulfurescens or Geotrichum candidum to afford ethyl (S)-3-hydroxy-3-phenylpropionate.[1] It undergoes Claisen condensation reaction with malononitrile to afford 2-cyano-5-phenyl-3,5-dioxopentanonitrile which on cyclization followed by coupling with diazonium salts yields azo derivatives.[2]
应用
包装
Packaged in glass bottles
储存分类代码
10 - Combustible liquids
WGK
WGK 2
闪点(°F)
284.0 °F - closed cup
闪点(°C)
140 °C - closed cup
个人防护装备
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
其他客户在看
Kuang-Po Chen et al.
Organic & biomolecular chemistry, 7(19), 4074-4081 (2009-09-19)
A manganese(III)-mediated reaction between 2-benzoyl-1,4-benzoquinones and 1,3-dicarbonyl compounds that produces benzo[c]furan-4,7-diones and anthracene-1,4-diones with high chemoselectivity is described. With ethyl butyrylacetate, by changing the solvent, benzo[c]furan-4,7-diones and anthracene-1,4-diones can be generated in high chemoselectivities. With ethyl benzoylacetate, N ,N-dimethyl acetoacetamide
Mohamed G Badrey et al.
Molecules (Basel, Switzerland), 17(10), 11538-11553 (2012-09-29)
A number of interesting heterocycles were prepared through interaction of the intermediate 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno-[2,3-d]pyrimidine (1) and reagents such as hydrazonyl halides 2 to furnish triazine derivatives 4a-l. Reaction of 1 with phenacyl bromide afforded compound 5. Moreover, the title compound 1
Asymmetric synthesis of both enantiomers of fluoxetine via microbiological reduction of ethyl benzoylacetate.
Chenevert R, et al.
Tetrahedron, 48(33), 6769-6776 (1992)
The reaction of ethyl benzoylacetate with malononitrile: a novel synthesis of some pyridazine, pyridazino [2, 3-a] quinazoline and pyrrole derivatives.
Abdelrazek FM, et al.
Tetrahedron, 57(9), 1813-1817 (2001)
Ugo Battaglia et al.
Journal of natural products, 73(11), 1938-1939 (2010-09-15)
A short synthesis of the 1,2,4-triazolo[1,5-a]pyrimidine antibiotic essramycin is described involving condensation of aminoguanidine with ethyl benzoylacetate to give an amino-1,2,4-triazole, followed by condensation with ethyl acetoacetate to form the pyrimidone ring.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持