选择尺寸
About This Item
推荐产品
方案
95%
表单
liquid
杂质
2-5% 4-methoxy-3-buten-2-one
折射率
n20/D 1.454 (lit.)
沸点
68-69 °C/14 mmHg (lit.)
密度
0.885 g/mL at 25 °C (lit.)
官能团
ether
储存温度
2-8°C
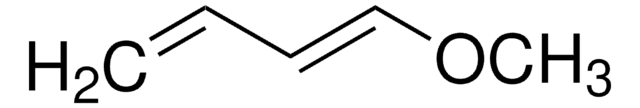
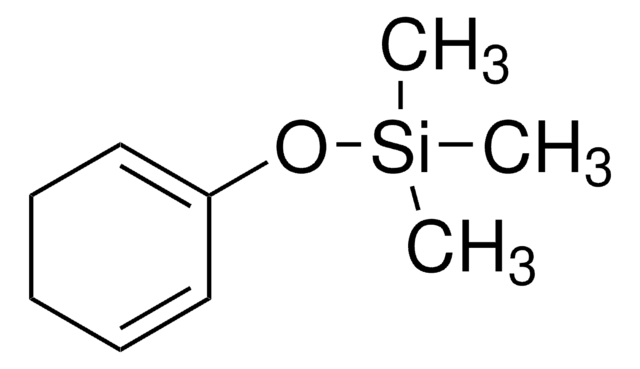
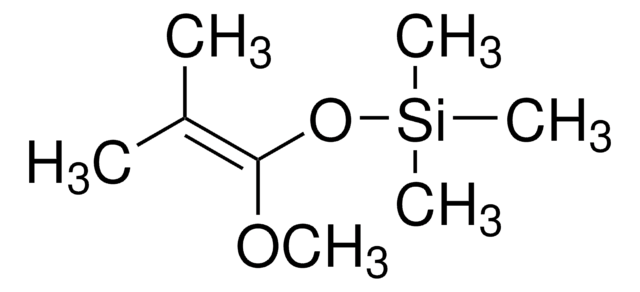
SMILES字符串
CO\C=C\C(=C)O[Si](C)(C)C
InChI
1S/C8H16O2Si/c1-8(6-7-9-2)10-11(3,4)5/h6-7H,1H2,2-5H3/b7-6+
InChI key
SHALBPKEGDBVKK-VOTSOKGWSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
警示用语:
Warning
危险声明
危险分类
Flam. Liq. 3
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
138.2 °F - closed cup
闪点(°C)
59 °C - closed cup
个人防护装备
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
其他客户在看
相关内容
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持