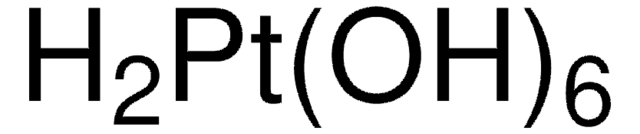Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
选择尺寸
About This Item
推荐产品
形狀
solid
品質等級
成份
Pt, 81-83%
反應適用性
core: platinum
reagent type: catalyst
環保替代產品特色
Designing Safer Chemicals
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
表面積
≥60 m2/g
mp
450 °C (lit.)
環保替代類別
, Aligned
SMILES 字串
O=[Pt]=O
InChI
1S/2O.Pt
InChI 密鑰
YKIOKAURTKXMSB-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
訊號詞
Danger
危險聲明
危險分類
Ox. Sol. 1
儲存類別代碼
5.1A - Strongly oxidizing hazardous materials
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
商品
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
相关内容
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis, and are some of the organic chemist’s most powerful tools for creating novel products. Below is a list of the most commonly used oxidizing and reducing agents currently available in our catalog.
-
What is the Department of Transportation shipping information for this product?
1 answer-
Helpful?
-
-
Is Product 206032, Platinum (IV) oxide, water soluble?
1 answer-
It is insoluble in water.
Helpful?
-
-
Are there any safety concerns with Product 206032, Platinum (IV) oxide?
1 answer-
After exposure to hydrogen, the resulting platinum black can be pyrophoric. Therefore, it should not be allowed to dry and all exposure to oxygen should be minimized.
Helpful?
-
-
How can Product 206032, Platinum (IV) oxide, be used as a catalyst?
1 answer-
Platinum(IV) oxide can be used as a catalyst in hydrogenations. The actual catalyst is platinum black which is formed in situ by reduction of the PtO2 by the hydrogen used for the hydrogenation. It is especially useful for reduction at room temperature and hydrogen pressures up to 4 atmospheres. It is suitable for the reduction of double and triple bonds, aromatic nuclei, carbonyl groups, nitro groups, and nitriles.
Helpful?
-
-
In which solvents is Product 206032, Platinum (IV) oxide, soluble?
1 answer-
Per the the chemicals encyclopedia published by the Royal Society of Chemistry, 13th Edition, when freshly precipitated, Platinum(IV) Oxide is soluble in concentrated acids and dilute phosphoric acid, especially when warmed. It is easily soluble in dilute solutions of potassium hydroxide.
Helpful?
-
-
Can the Platinum be recovered from Product 206032, Platinum (IV) oxide?
1 answer-
Internet sources do indicate that Platinum can be recovered from spent catalyst by conversion to ammonium chloroplatinate using aqua regia followed by ammonia. However, we do have a process for doing so.
Helpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门