推荐产品
方案
98%
折射率
n20/D 1.539 (lit.)
沸点
77 °C/0.01 mmHg (lit.)
密度
1.091 g/mL at 25 °C (lit.)
官能团
ketone
phenyl
储存温度
2-8°C
SMILES字符串
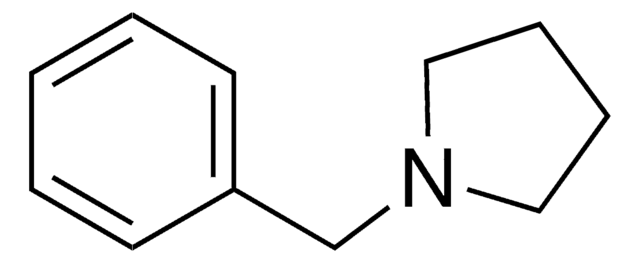
O=C1CCN(C1)Cc2ccccc2
InChI
1S/C11H13NO/c13-11-6-7-12(9-11)8-10-4-2-1-3-5-10/h1-5H,6-9H2
InChI key
DHGMDHQNUNRMIN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
10 - Combustible liquids
WGK
WGK 3
闪点(°F)
230.0 °F - closed cup
闪点(°C)
110 °C - closed cup
个人防护装备
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
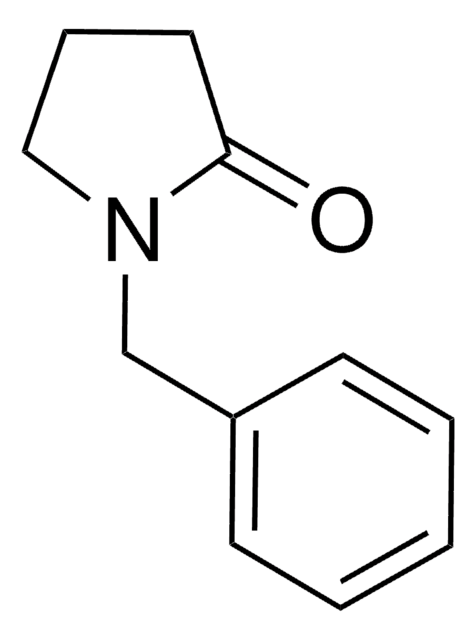
Facile synthesis of 3-arylpyrroles by tandem Suzuki-dehydrogenation reaction.
Lee C-W and Chung YJ.
Tetrahedron Letters, 41(18), 3423-3425 (2000)
Synthesis, 2224-2224 (2006)
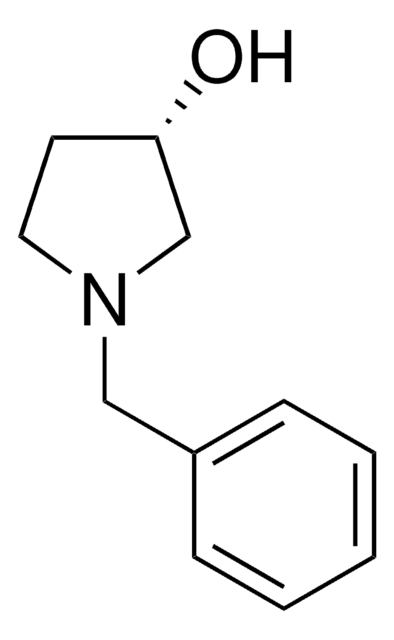
Practical synthetic process for enantiopure 1-benzyl-3-hydroxypyrrolidine.
Morimoto M and Sakai K.
Tetrahedron Asymmetry, 19(12), 1465-1469 (2008)
A thermodynamic study of ketoreductase-catalyzed reactions 5. Reduction of substituted ketones in n-hexane.
Tewari YB, et al.
The Journal of Chemical Thermodynamics, 40(4), 661-670 (2008)
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持