If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdf
推荐产品
质量水平
方案
97%
表单
liquid
折射率
n20/D 1.607 (lit.)
沸点
110-112 °C/20 mmHg (lit.)
mp
7 °C (lit.)
密度
1.427 g/mL at 25 °C (lit.)
官能团
bromo
phenyl
储存温度
2-8°C
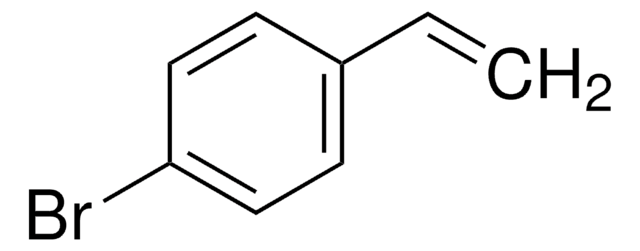
SMILES字符串
Br\C=C\c1ccccc1
InChI
1S/C8H7Br/c9-7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
YMOONIIMQBGTDU-VOTSOKGWSA-N
正在寻找类似产品? 访问 产品对比指南
其他客户在看
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
Helpful?
-
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
Helpful?
-
-
WHAT IS THE STEREOCHEMISTRY OF THIS COMPOUND?
1 answer-
The entire product is likely planar due to the conjugated effect between the aromatic ring and C=C double bond.
Helpful?
-
-
is there any possibility for decreacing the purity of the compound with out innert gases storage???
1 answer-
Yes, the purity of may decline over time if exposed to atmospheric oxygen. Storing the compound under inert gas, such as nitrogen or argon, prevents decomposition. Please see the link below to review additional information regarding the breakdown reaction published to PubChem:
https://pubchem.ncbi.nlm.nih.gov/compound/5314126#section=Non-Human-Toxicity-ValuesHelpful?
-
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持