15408
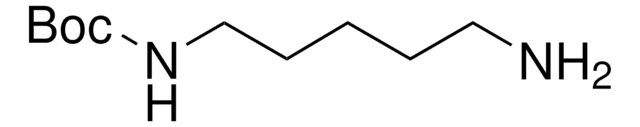
N-Boc-1,3-propanediamine
≥97.0% (GC/NT)
Sinónimos:
N-Boc-1,3-diaminopropane, tert-Butyl N-(3-aminopropyl)carbamate
About This Item
Productos recomendados
Quality Level
assay
≥97.0% (GC/NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.454 (lit.)
n20/D 1.459
bp
203 °C (lit.)
mp
22 °C (lit.)
density
0.998 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCNC(OC(C)(C)C)=O
InChI
1S/C8H18N2O2/c1-8(2,3)12-7(11)10-6-4-5-9/h4-6,9H2,1-3H3,(H,10,11)
InChI key
POHWAQLZBIMPRN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Tackling vancomycin-resistant bacteria with ′lipophilic–vancomycin–carbohydrate conjugates′: This study discusses the synthesis of derivatives using N-Boc-1,3-propanediamine to develop new antibacterial agents targeting resistant bacterial strains (Yarlagadda et al., 2015).
- Sulfonamides differing in the alkylamino substituent length–Synthesis, electrochemical characteristic, acid-base profile and complexation properties: The study involves N-Boc-1,3-propanediamine in the synthesis of novel sulfonamide derivatives with potential biochemical applications (Ciesielska et al., 2022).
- Direct α-alkylation of primary aliphatic amines enabled by CO2 and electrostatics: Research demonstrating selective α-alkylation of N-Boc-1,3-propanediamine, highlighting a novel method in organic synthesis (Ye et al., 2018).
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
228.2 °F - closed cup
flash_point_c
109 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico