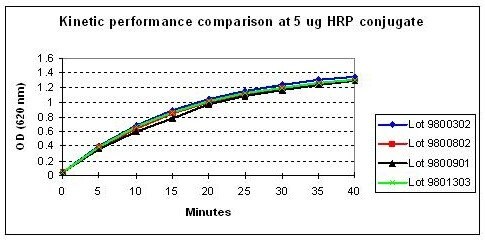
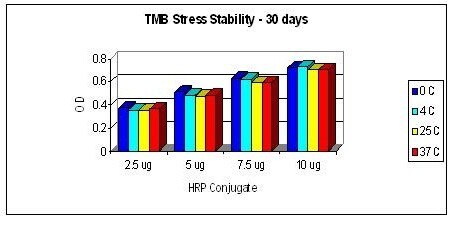
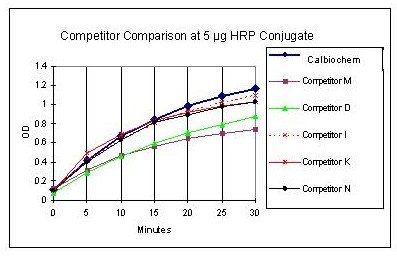
This product is a ready to use product. The addition of hydrogen peroxide is not required. For use in Elisa, please see the general protocol below:
1. Complete all required incubations with antibodies and HRP-labeled probes.
2. Wash plate wells at least 4 times with phosphate-buffered saline (PBS) or Tris-buffered saline (TBS), containing 0.1% Tween®-20 detergent.
3. After the final wash, shake and blot all residual buffer from the wells.
4. Add 100 µl TMB solution to each well and incubate for 5-30 min.
5. Allow the substrate reaction to develop a blue soluble reaction by incubating for the optimal time determined for the assay.
6. The reaction should be stopped by using a 450 nm or 650 nm stop solution.
7. Measure the absorbance at 450 nm or 650 nm of each microwell within 1 hour.
8. Options for measurements:
a. For kinetic assays, the reaction can be monitored as a function of time by reading absorbance at 650 nm at intermediate intervals.
b. For endpoint assays that preserve the blue chromogen, the reaction should be stopped by addition of 100 µl of 0.1% sodium fluoride (NaF) and the absorbance read at 650 nm.
c. If increased sensitivity is desired for endpoint assays, the reaction should be stopped by addition of 100 µl of either 500 mM H2SO4 or 250 mM HCl and the absorbance read at 450 nm WITHIN 5 MIN. Addition of acid converts the blue radical to the yellow diimine, which absorbs at 450 nm.
NOTE: Optimal incubation times may vary depending on the amount of HRP present. If color develops too quickly, zero-order kinetics will not prevail. Dilution of the probe, antibody, or HRP-labeled reagent may be required. Variations in time, reagent volumes, and temperature may also require further standardization by the user.