About This Item
Recommended Products
Assay
95%
reaction suitability
reagent type: oxidant
mp
220-222 °C (lit.)
functional group
amine
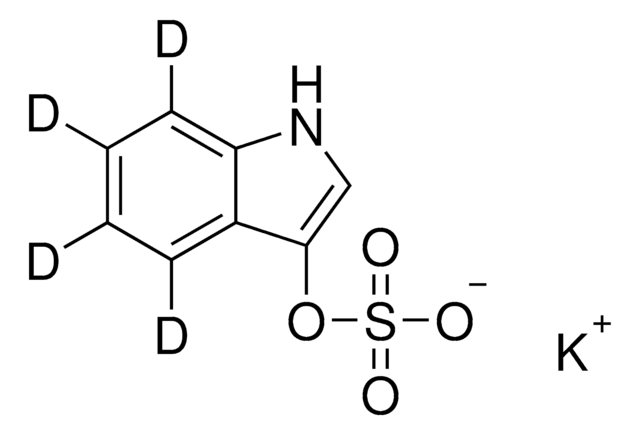
SMILES string
C[N+](C)(C)[O-]
InChI
1S/C3H9NO/c1-4(2,3)5/h1-3H3
InChI key
UYPYRKYUKCHHIB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a demetallation and decarbonylation reagent for organometallic compounds.
- To prepare azomethine ylide by reaction with lithium di-isopropylamide. This, in turn, may be reacted with simple alkenes to obtain corresponding pyrrolidines.
- To mediate the conversion of thiols to disulfides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service