所有图片(1)
About This Item
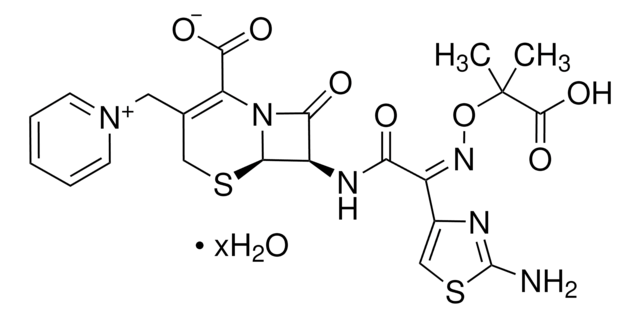
经验公式(希尔记法):
C22H22N6O7S2 · 5H2O
CAS号:
分子量:
636.65
MDL编号:
UNSPSC代码:
41116107
NACRES:
NA.24
价格与库存信息目前不能提供
推荐产品
等级
pharmaceutical primary standard
API类
ceftazidime
制造商/商品名称
USP
应用
pharmaceutical (small molecule)
包装形式
neat
储存温度
−20°C
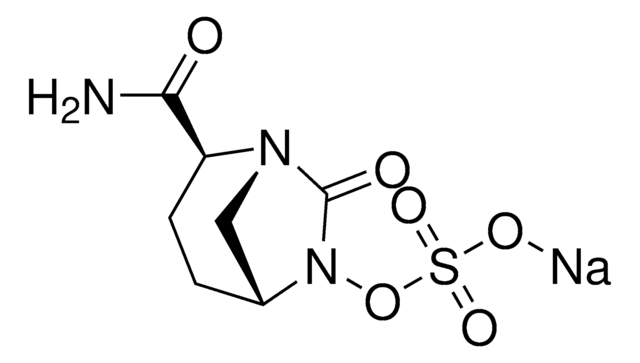
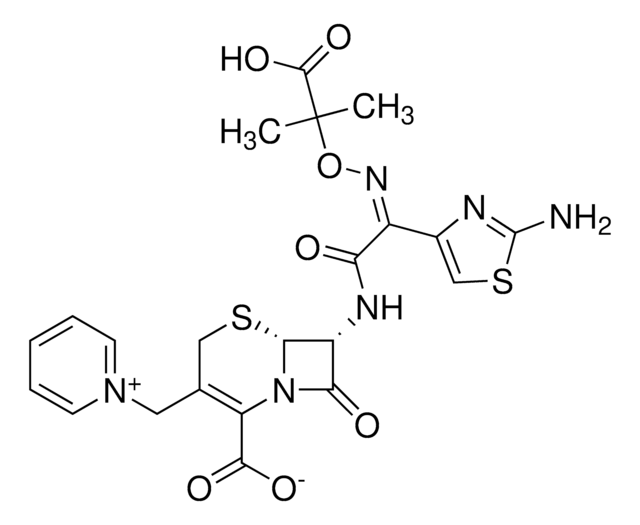
SMILES字符串
[s]1c(nc(c1)\C(=N\OC(C)(C)C(=O)[O-])\C(=O)N[C@H]2[C@H]3SCC(=C(N3C2=O)C(=O)[O-])C[n+]4ccccc4)N.O.O.O.O.O.[H+]
InChI
1S/C22H22N6O7S2.5H2O/c1-22(2,20(33)34)35-26-13(12-10-37-21(23)24-12)16(29)25-14-17(30)28-15(19(31)32)11(9-36-18(14)28)8-27-6-4-3-5-7-27;;;;;/h3-7,10,14,18H,8-9H2,1-2H3,(H4-,23,24,25,29,31,32,33,34);5*1H2/b26-13-;;;;;/t14-,18-;;;;;/m1...../s1
InChI key
NMVPEQXCMGEDNH-TZVUEUGBSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
应用
Ceftazidime pentahydrate USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Ceftazidime
- Ceftazidime Injection
- Ceftazidime for Injection
分析说明
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他说明
Sales restrictions may apply.
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
其他客户在看
Ceftazidime
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 45(3), 872-872 (2018)
Kai Ming Chow et al.
Pharmacotherapy, 23(3), 369-373 (2003-03-12)
We reviewed 42 cases of cefepime-induced neurotoxicity and 12 cases of ceftazidime-induced neurotoxicity from the literature and our institution. Clinical characteristics and timing of diagnosis were examined. Common findings were confusion with temporospatial disorientation (96% of patients), myoclonus (33%), and
Jason A Roberts et al.
International journal of antimicrobial agents, 29(2), 117-128 (2006-12-13)
Sepsis and nosocomial infections continue to be a significant problem in intensive care, contributing heavily to mortality and prolonged hospital stay. Early and appropriate antibiotic therapy is critical for optimising outcomes. However, the emergence of highly resistant bacteria, coupled with
S Segal-Maurer et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 28(5), 1134-1138 (1999-08-19)
Increasing prevalence of multidrug-resistant gram-negative organisms has led to a rise in clinically significant infections with these organisms and an increasing therapeutic dilemma. We present a case of a neurosurgical patient who developed ventriculoperitoneal shunt-associated ventriculitis due to ceftazidime-resistant Klebsiella
M A Sirgo et al.
DICP : the annals of pharmacotherapy, 25(3), 284-288 (1991-03-01)
The disposition of drugs in the elderly is particularly relevant with antiinfectives, because this population has an increased risk of infections. Renal function deteriorates with age, yet dosage guidelines for antibiotics that allow for this reduction remain to be established.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门