SAE0090
Beta-galactoside alpha-2,6-sialyltransferase 1
≥300 units/mg protein, ST6GAL1 human recombinant, expressed in HEK 293 cells
别名:
Alpha 2,6-ST 1, B-cell antigen CD75, CMP-N-acetylneuraminate-beta-galactosamide-alpha-2,6-sialyltransferase 1, ST6Gal I, Sialyltransferase 1
About This Item
推荐产品
重组
expressed in HEK 293 cells
描述
The specific activity of ST6Gal I is measured by its ability to transfer sialic acid from CMP-NANA to asialofetuin.
方案
≥95% (SDS-PAGE)
表单
lyophilized powder
比活
≥300 units/mg protein
运输
ambient
储存温度
−20°C
相关类别
一般描述
生化/生理作用
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity,3 as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.4-5
This recombinant ST6Gal I product can be used to study the mode of action of the enzyme, as well as its potential inhibitors. It can also be used as a glycoengineering tool to modify glycoproteins in vitro.
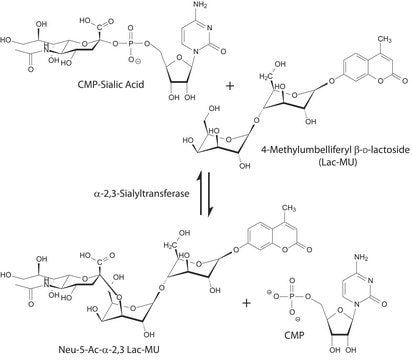
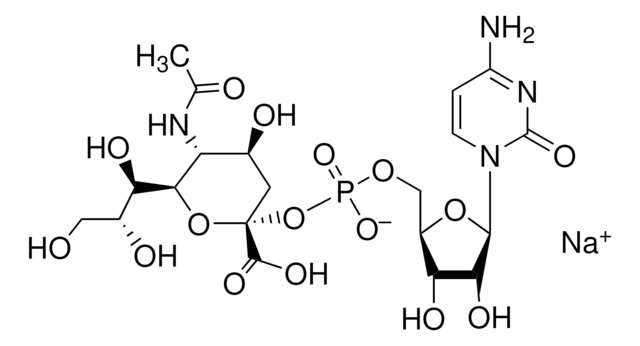
CMP-N-acetylneuraminate (CMP-sialic acid, CMP-NANA) to the β-D-galactosyl-1,4-N-acetyl-D-glucosaminyl termini on glycoproteins.
Sialic acids are distributed in a variety of glycolipids and glycoproteins. The sialic acid that is added to a galactose (Gal) can be bound either to the hydroxyl attached to carbon-3 of Gal to form an α-2,3 glycosidic linkage, or to the hydroxyl group attached to carbon-6 to form an α-2,6 glycosidic linkage. ST6Gal I generates a α-2,6 linkage of sialic acid on the non-reducing, terminal Galβ1 4GlcNAc residues of oligosaccharides and glycoconjugates.
Terminal sialylation has been shown to decrease Fcγ receptor binding and increase anti-inflammatory activity, as well as antibody-dependent cellular cytotoxicity in different studies by reduced binding of sialylated antibody towards FcγRIIIa.
单位定义
储存分类代码
11 - Combustible Solids
WGK
WGK 2
闪点(°F)
Not applicable
闪点(°C)
Not applicable
历史批次信息供参考:
商品
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Explore tools for glycosyltransferase synthesis and modification of glycans, such as glycosyltransferases and nucleotide sugar donors.
Active Filters
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门