推荐产品
生物源
synthetic
產品線
BioReagent
形狀
powder or crystals
效力
845-988 μg per mg (C16H18N3O4S, Calculated on the anhydrous basis)
技術
cell culture | mammalian: suitable
顏色
white to off-white
mp
215 °C (dec.) (lit.)
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
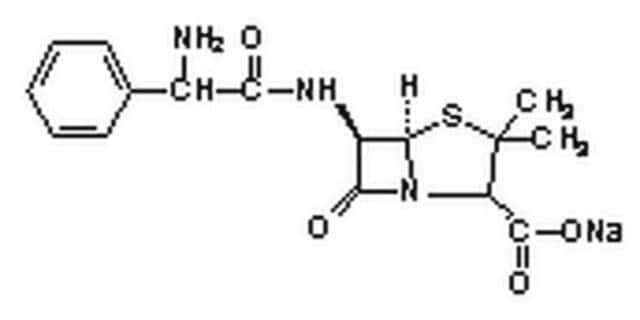
SMILES 字串
[Na+].CC1(C)SC2[C@H](NC(=O)[C@H](N)c3ccccc3)C(=O)N2[C@H]1C([O-])=O
InChI
1S/C16H19N3O4S.Na/c1-16(2)11(15(22)23)19-13(21)10(14(19)24-16)18-12(20)9(17)8-6-4-3-5-7-8;/h3-7,9-11,14H,17H2,1-2H3,(H,18,20)(H,22,23);/q;+1/p-1/t9-,10-,11+,14-;/m1./s1
InChI 密鑰
KLOHDWPABZXLGI-YWUHCJSESA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
Ampicillin sodium salt, belonging to the extended-spectrum β-lactam family, stands as a semisynthetic derivative of penicillin with versatile applications as a broad-spectrum antibiotic. It exerts inhibitory effects on bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPs), thereby impeding peptidoglycan synthesis—an integral process in the formation of the bacterial cell wall. This antibiotic demonstrates activity against an extensive spectrum of bacteria, encompassing both Gram-positive and Gram-negative strains such as E. coli, β-lactam sensitive vancomycin-resistant Enterococcus (VRE), Staphylococcus aureus, and Streptococcus pneumoniae.
In research, ampicillin plays a pivotal role in microbiological, biochemical, and cell culture investigations. Its utilization in laboratories extends to studying antibiotic resistance and penetration limitations, exploring the synergistic interactions between multiple antibiotics, and serving as a crucial component for the selection and maintenance of recombinant plasmids in E. coli. Through these applications, ampicillin sodium salt contributes significantly to advancing the understanding of antibiotic efficacy, bacterial responses, and molecular processes, making it an indispensable tool in various facets of scientific research.
In research, ampicillin plays a pivotal role in microbiological, biochemical, and cell culture investigations. Its utilization in laboratories extends to studying antibiotic resistance and penetration limitations, exploring the synergistic interactions between multiple antibiotics, and serving as a crucial component for the selection and maintenance of recombinant plasmids in E. coli. Through these applications, ampicillin sodium salt contributes significantly to advancing the understanding of antibiotic efficacy, bacterial responses, and molecular processes, making it an indispensable tool in various facets of scientific research.
應用
建议以 100mg/L 的抗菌用量用于细胞培养基中。
建议以 20-125μg/ml 的量用于氨苄西林抗性研究。
在 37°C 下可稳定 3 天。
建议以 20-125μg/ml 的量用于氨苄西林抗性研究。
在 37°C 下可稳定 3 天。
生化/生理作用
作用方式:该ß-内酰胺抗生素通过灭活位于细菌的细胞膜内表面的转肽酶从而抑制细菌细胞壁合成。
耐药性机制:ß-内酰胺酶切开氨苄青霉素的ß-内酰胺环,使其失去活性。
抗菌谱:包括革兰氏阳性菌(类似青霉素)和革兰氏阴性菌(类似四环素和氯霉素)。
耐药性机制:ß-内酰胺酶切开氨苄青霉素的ß-内酰胺环,使其失去活性。
抗菌谱:包括革兰氏阳性菌(类似青霉素)和革兰氏阴性菌(类似四环素和氯霉素)。
特點和優勢
- High quality antibiotic suitable for multiple research applications
- Broad-spectrum antibiotic
- Inhibits bacterial cell-wall synthesis
- Active against Gram-positive and Gram-negative bacteria
- Commonly used in Cell Culture, Cell Biology and Biochemical research
包裝
5g, 25g, 100g
注意
This product has been reported stable as supplied at 25°C at 43% and 81% relative humidity for six weeks. It is stable at 37°C for three days. Additional studies have shown that the stability of Ampicillin in solution is a function of pH, temperature and the identity of the buffer. It′s activity is quickly lost when stored above pH 7. Optimal storage conditions are suggested as 2-8°C, and pH 3.8-5 where its activity was retained at 90%+ for a week.
準備報告
Ampicillin is reported as slightly soluble in water, practically insoluble in alcohol, chloroform, ether and fixed oils but soluble in dilute acids or bases. The solution should not be autoclaved; a stock solution should be sterilized through filtration and stored frozen, where it will be stable for months.
儲存和穩定性
Tightly closed. Dry. Keep locked up or in an area accessible only to qualified or authorized
其他說明
For additional information on our range of Biochemicals, please complete this form.
Keep container tightly closed in a dry and well-ventilated place. Keep in a dry place.
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1A - Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
M Ashley Sobran et al.
Journal of bacteriology, 201(17) (2019-06-27)
To detect and respond to the diverse environments they encounter, bacteria often use two-component regulatory systems (TCS) to coordinate essential cellular processes required for survival. In pathogenic Bordetella species, the BvgAS TCS regulates expression of hundreds of genes, including those
Alexander Frank et al.
Current biology : CB, 28(16), 2597-2606 (2018-08-07)
Synchronization of circadian clocks to the day-night cycle ensures the correct timing of biological events. This entrainment process is essential to ensure that the phase of the circadian oscillator is synchronized with daily events within the environment [1], to permit
Nuria Fernández-Hidalgo et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(9), 1261-1268 (2013-02-09)
The aim of this study was to compare the effectiveness of the ampicillin plus ceftriaxone (AC) and ampicillin plus gentamicin (AG) combinations for treating Enterococcus faecalis infective endocarditis (EFIE). An observational, nonrandomized, comparative multicenter cohort study was conducted at 17
Sabine Renggli et al.
Journal of bacteriology, 195(18), 4067-4073 (2013-07-10)
Bactericidal antibiotics kill by different mechanisms as a result of a specific interaction with their cellular targets. Over the past few years, alternative explanations for cidality have been proposed based on a postulated common pathway, depending on the intracellular production
Daejin Lim et al.
Frontiers in bioengineering and biotechnology, 8, 840-840 (2020-08-09)
Targeted delivery of drugs is a key aspect of the successful treatment of serious conditions such as tumors. In the pursuit of accurate delivery with high specificity and low size limit for peptide drugs, a synthetic type 3 secretion system
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门