推荐产品
生物来源
plant (Peumus boldus molina)
质量水平
等级
analytical standard
方案
≥98% (TLC)
技术
HPLC: suitable
gas chromatography (GC): suitable
杂质
≤1% isopropanol
mp
157-164 °C
溶解性
ethanol: 50 mg/mL
应用
food and beverages
forensics and toxicology
veterinary
包装形式
neat
储存温度
room temp
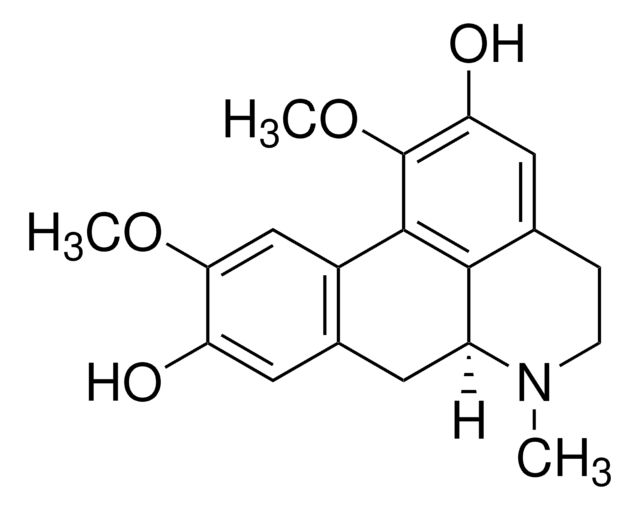
SMILES字符串
COc1cc-2c(CC3N(C)CCc4cc(O)c(OC)c-2c34)cc1O
InChI
1S/C19H21NO4/c1-20-5-4-10-7-15(22)19(24-3)18-12-9-16(23-2)14(21)8-11(12)6-13(20)17(10)18/h7-9,13,21-22H,4-6H2,1-3H3/t13-/m0/s1
InChI key
LZJRNLRASBVRRX-ZDUSSCGKSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
Boldine is an aporphine alkaloid isolated from Boldo tree and other plant species. It is a free radical scavenger with anti-inflammatory activity while exhibiting other pharmacological effects such as antidiabetic, antiplatelet aggregation, antipyretic, antinociceptive, antiatherogenic, hepatoprotective and endothelium-protective activity.
应用
Boldine may be used as an analytical reference standard for the quantification of the analyte in biological samples and pharmaceutical preparations using chromatography techniques.
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
警示用语:
Warning
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 1
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Mat Ropi Mukhtar et al.
Molecules (Basel, Switzerland), 14(3), 1227-1233 (2009-03-28)
The stem bark of Phoebe grandis afforded one new oxoproaporphine; (-)-grandine A (1), along with six known isoquinoline alkaloids: (-)-8,9-dihydrolinearisine (2), boldine, norboldine, lauformine, scortechiniine A and scortechiniine B. In addition to that of the new compound, complete 1H- and
Eduardo L Konrath et al.
Neurotoxicology, 29(6), 1136-1140 (2008-07-02)
Boldine is one of the most potent natural antioxidants and displays some important pharmacological activities, such as cytoprotective and anti-inflammatory activities, which may arise from its free radical scavenging properties. Given that the pathogenesis of brain ischemia/reperfusion has been associated
Franz A Thomet et al.
Molecules (Basel, Switzerland), 16(3), 2253-2258 (2011-03-09)
2,9-Dimethoxymethylboldine (2), 2,9-dimethoxymethyl-3-bromoboldine (3) and 2,9-dimethoxymethyl-3-diphenylphosphinylboldine (4) have been synthesized in an effort to find compounds with potential pharmacological applications. The cytotoxic activities of the natural precursor 1 and these three derivatives have been measured as IC₅₀ inhibitory growth. The
HPLC analysis of boldine in pharmaceuticals
Orsi D.D, et al.
Chromatographia, 44(11-12), 610-622 (1997)
Advances in development of dopaminergic aporphinoids.
Ao Zhang et al.
Journal of medicinal chemistry, 50(2), 171-181 (2007-01-19)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门