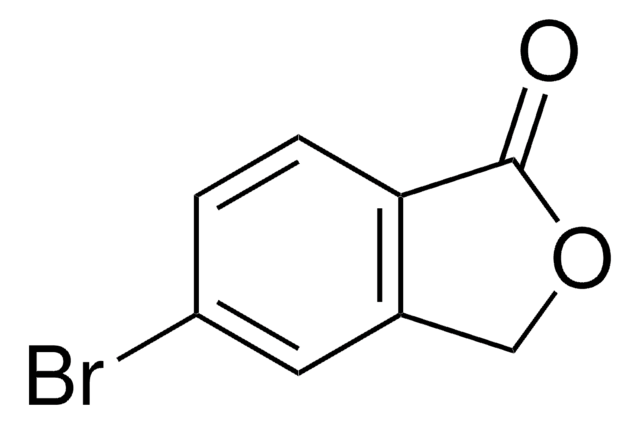
推荐产品
品質等級
化驗
98%
形狀
powder
bp
290 °C (lit.)
mp
71-74 °C (lit.)
SMILES 字串
O=C1OCc2ccccc12
InChI
1S/C8H6O2/c9-8-7-4-2-1-3-6(7)5-10-8/h1-4H,5H2
InChI 密鑰
WNZQDUSMALZDQF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
305.6 °F - closed cup
閃點(°C)
152 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Tsz-Ying Yuen et al.
Organic letters, 14(19), 5154-5157 (2012-09-29)
A highly convergent total synthesis of 7',8'-dihydroaigialospirol is described. Key steps of the synthesis include a Nozaki-Hiyama-Kishi (NHK) coupling of an iodoalkyne with an advanced phthalide-aldehyde and a remarkable one-pot acid-mediated global deprotection/spiroacetalization.
Vatcharin Rukachaisirikul et al.
Journal of natural products, 75(5), 853-858 (2012-04-25)
Nine new fungal metabolites, one phthalide derivative, acremonide (1), and eight isocoumarin derivatives, acremonones A-H (2-9), were isolated from the mangrove-derived fungus Acremonium sp. PSU-MA70 together with 10 known compounds. Their structures were determined by NMR analysis. The known 8-deoxytrichothecin
R Santhosh Reddy et al.
Organic & biomolecular chemistry, 10(18), 3655-3661 (2012-04-13)
The asymmetric dihydroxylation (AD) of o-cyano cinnamates and styrene derivatives leads to efficient construction of chiral phthalide frameworks in high optical purities. This unique reaction is characterized by unusual synergism between CN and osmate groups resulting in rate enhancement of
Jaganathan Karthikeyan et al.
Chemical communications (Cambridge, England), 47(37), 10461-10463 (2011-08-19)
A cobalt-catalyzed addition of aryl- and alkenylboronic acids to aldehydes and phthalaldehyde to give the corresponding biarylketones and 3-aryl phthalides in good to excellent yields in one pot is described.
Juan Mangas-Sánchez et al.
Organic letters, 14(6), 1444-1447 (2012-03-08)
A straightforward synthesis of (S)-3-methylphthalides has been developed, with the key asymmetric step being the bioreduction of 2-acetylbenzonitriles. Enzymatic processes have been found to be highly dependent on the pH value, with acidic conditions being required to avoid undesired side
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门