推荐产品
化驗
≥95% (HPLC)
形狀
powder
儲存溫度
2-8°C
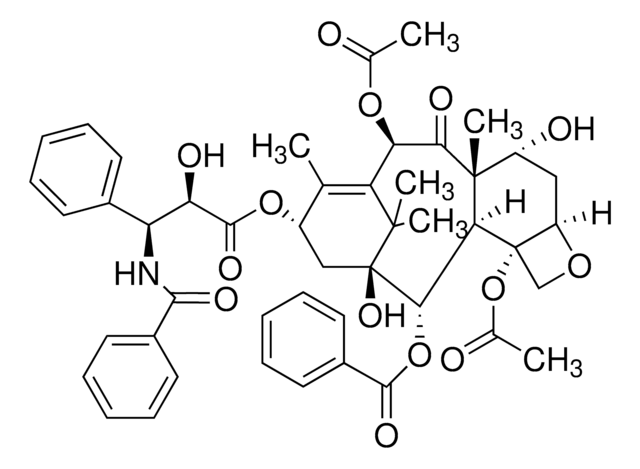
SMILES 字串
CC(=O)O[C@H]1C(=O)[C@]2(C)[C@@H](O)C[C@H]3OC[C@@]3(OC(C)=O)[C@H]2[C@H](OC(=O)c4ccccc4)[C@]5(O)C[C@H](O)C(C)=C1C5(C)C
InChI
1S/C31H38O11/c1-15-19(34)13-31(38)26(41-27(37)18-10-8-7-9-11-18)24-29(6,20(35)12-21-30(24,14-39-21)42-17(3)33)25(36)23(40-16(2)32)22(15)28(31,4)5/h7-11,19-21,23-24,26,34-35,38H,12-14H2,1-6H3/t19-,20-,21+,23+,24-,26-,29+,30-,31+/m0/s1
InChI 密鑰
OVMSOCFBDVBLFW-VHLOTGQHSA-N
正在寻找类似产品? 访问 产品对比指南
應用
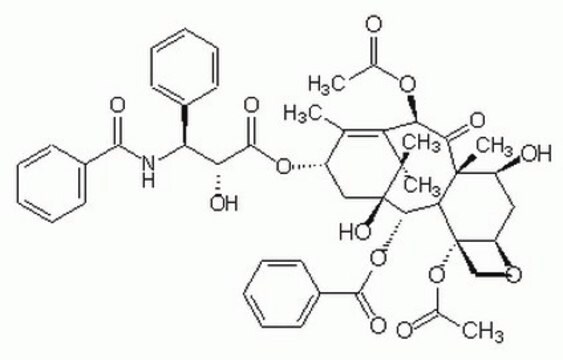
紫杉酚的前体
訊號詞
Danger
危險分類
Carc. 1B - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Catherine Loncaric et al.
Chemistry & biology, 13(3), 309-317 (2006-04-28)
The 10beta-acetyltransferase on the biosynthetic pathway of the antineoplastic drug Taxol catalyzes the regiospecific transfer of the acetyl group of acetyl-coenzyme A (CoA) to 10-deacetylbaccatin III. We demonstrate that in addition to acetyl group transfer, the overexpressed enzyme also catalyzes
Xin Che et al.
Journal of pharmaceutical and biomedical analysis, 55(5), 1190-1196 (2011-05-03)
The isolation and characterization of the process related impurities and degradation products of larotaxel drug substance were described. Forced degradation of larotaxel was carried out under acidic, basic, oxidation, light and thermal conditions to assess the nature of the impurities.
Young-Hee Lee et al.
International immunopharmacology, 21(2), 487-493 (2014-06-25)
Myeloid-derived suppressor cells (MDSCs) mediate tumor-associated immune suppression in both cancer patients and tumor-bearing animals. Reduction or elimination of MDSCs reduces the rate of tumor progression and improves cancer therapies that employ mechanisms of immunity. Here we show that baccatin
Mohammad K Parvez et al.
Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society, 27(3), 389-400 (2019-04-13)
Despite high anti-HBV efficacies, while the nucleoside analogs (e.g., lamivudine) lead to the emergence of drug-resistance, interferons (e.g., IFN-α causes adverse side-effects. Comparatively, various natural or plant products have shown similar or even better efficacy. Hence, new antiviral strategies must
Mark E Ondari et al.
The Journal of organic chemistry, 74(5), 2186-2188 (2009-02-10)
A one-pot trisilylation step to protect three hydroxyl groups of baccatin III (1), followed by hydride ester cleavage and base hydrolysis of a triethylsilyl ether at C13, provides efficient access to a key intermediate 9 (top path). This route removes
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门