About This Item
推荐产品
方案
95%
表单
powder
反应适用性
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
205-208 °C
SMILES字符串
[CH2][CH][CH]c1ccccc1.CC(C)c2cccc(C(C)C)c2N3C=CN(\C3=[Pd]/Cl)c4c(cccc4C(C)C)C(C)C
InChI
1S/C27H36N2.C9H9.ClH.Pd/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;1-2-6-9-7-4-3-5-8-9;;/h9-16,18-21H,1-8H3;2-8H,1H2;1H;/q;;;+1/p-1
InChI key
RWGNZUQSYXTZIX-UHFFFAOYSA-M
相关类别
应用
- Catalyst for Suzuki coupling (eq. 1)
- Catalyst for Buchwald-Hartwig amination reaction (eq. 2)
- Suzuki-Miyaura couplings
- Buchwald-Hartwig couplings
法律信息
警示用语:
Warning
危险分类
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
储存分类代码
13 - Non Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门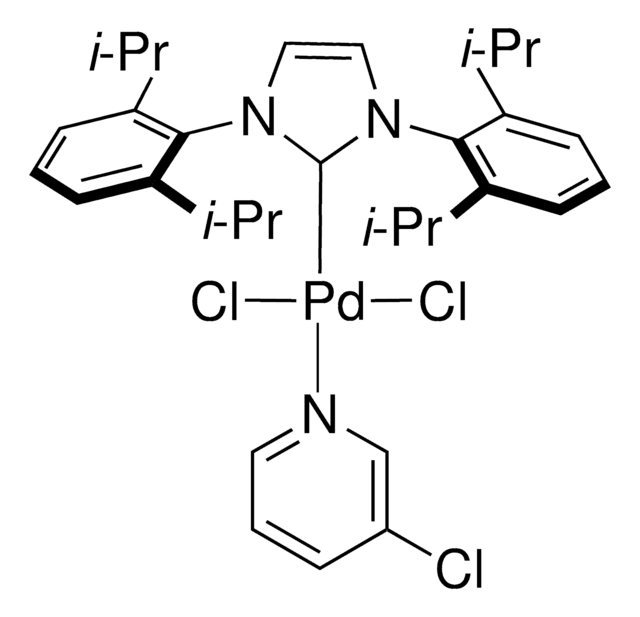

![[Pd(IPr#)(cin)Cl]](/deepweb/assets/sigmaaldrich/product/structures/391/578/9bb7eaef-fa70-4f50-8644-2c55cec3925d/640/9bb7eaef-fa70-4f50-8644-2c55cec3925d.png)
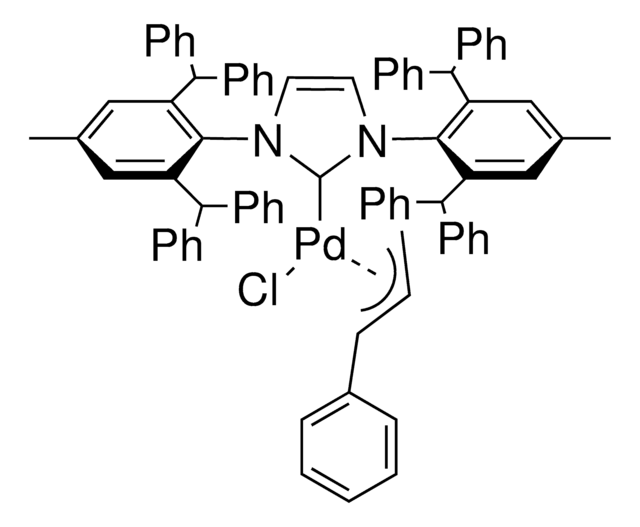


![[1,3-双(2,6-二-异丙基苯基)-4,5-二氢咪唑-2-亚基]氯][3-苯基烯丙基]钯(II) Umicore, 95%](/deepweb/assets/sigmaaldrich/product/structures/323/737/7e145d9c-5a23-4d5d-95f8-8e2d2fa5b255/640/7e145d9c-5a23-4d5d-95f8-8e2d2fa5b255.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![[(IPr)AuCl] Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
![氯[1,3-双(2,6-二异丙基苯基)咪唑-2-亚基]铜(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)


