所有图片(1)
About This Item
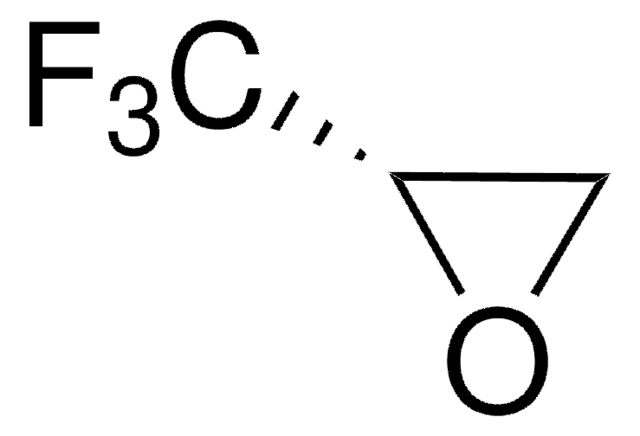
经验公式(希尔记法):
C3H3F3O
CAS号:
分子量:
112.05
MDL编号:
UNSPSC代码:
12352005
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
97%
折射率
n20/D <1.300
沸点
25-32 °C
密度
1.294 g/mL at 25 °C
官能团
ether
fluoro
储存温度
2-8°C
SMILES字符串
FC(F)(F)[C@H]1CO1
InChI
1S/C3H3F3O/c4-3(5,6)2-1-7-2/h2H,1H2/t2-/m1/s1
InChI key
AQZRARFZZMGLHL-UWTATZPHSA-N
应用
(R)-(+)-3,3,3-Trifluoro-1,2-epoxypropane can be used as a substrate to synthesize:
- Substituted trifluoro amino propanols, which are found to be potent inhibitors of cholesteryl ester transfer protein.
- (2R) Trifluoro-(methoxybenzyloxy)-propanol (chiral glycol) by reacting with 4-methoxybenzyl alcohol in the presence of NaH. Chiral glycol intermediate is further utilized for the preparation of trifluoromethyl glycol carbamates as potential monoacylglycerol lipase (MAGL) inhibitors.
警示用语:
Danger
危险声明
预防措施声明
危险分类
Flam. Liq. 1
储存分类代码
3 - Flammable liquids
WGK
WGK 3
闪点(°F)
-14.8 °F
闪点(°C)
-26 °C
个人防护装备
Eyeshields, Faceshields, Gloves
Discovery of a simple picomolar inhibitor of cholesteryl ester transfer protein
Reinhard EJ, et al.
Journal of Medicinal Chemistry, 46(11), 2152-2168 (2003)
Discovery of trifluoromethyl glycol carbamates as potent and selective covalent monoacylglycerol lipase (MAGL) inhibitors for treatment of neuroinflammation
McAllister LA, et al.
Journal of Medicinal Chemistry, 61(7), 3008-3026 (2018)
Emily J Reinhard et al.
Journal of medicinal chemistry, 46(11), 2152-2168 (2003-05-16)
A novel series of substituted N-[3-(1,1,2,2-tetrafluoroethoxy)benzyl]-N-(3-phenoxyphenyl)-trifluoro-3-amino-2-propanols is described which potently and reversibly inhibit cholesteryl ester transfer protein (CETP). Starting from the initial lead 1, various substituents were introduced into the 3-phenoxyaniline group to optimize the relative activity for inhibition of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门