所有图片(2)
About This Item
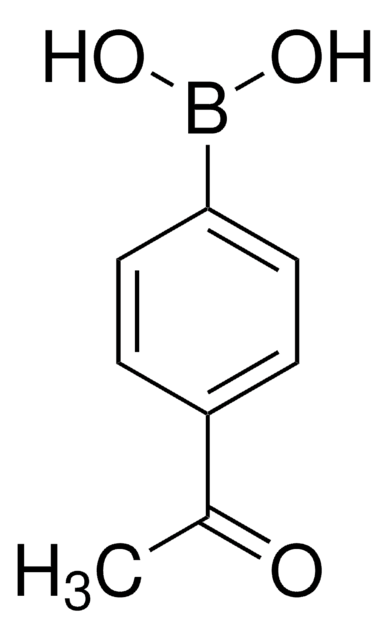
线性分子式:
CH3COC6H4B(OH)2
CAS号:
分子量:
163.97
MDL编号:
UNSPSC代码:
12352103
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
≥95%
杂质
<10% 3-acetylphenylboronic anhydride
mp
204-208 °C (lit.)
官能团
ketone
SMILES字符串
CC(=O)c1cccc(c1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-3-2-4-8(5-7)9(11)12/h2-5,11-12H,1H3
InChI key
SJGGDZCTGBKBCK-UHFFFAOYSA-N
应用
3-Acetylphenylboronic acid can be used as a substrate:
- In the synthesis of symmetric biaryls via oxidative dimerization using a palladium catalyst and water as a solvent.
- In the synthesis of aryl fluorides through electrophilic fluorination reaction using acetyl hypofluorite.
- In the coupling reactions of organoboranes with olefins using molecular oxygen and palladium catalyst.
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Fluorination of aryl boronic acids using acetyl hypofluorite made directly from diluted fluorine
Vints I, et al.
The Journal of Organic Chemistry, 78(23), 11794-11797 (2013)
Oxygen-promoted Pd (II) catalysis for the coupling of organoboron compounds and olefins
Jung YC, et al.
Organic Letters, 5(13), 2231-2234 (2003)
Oxidative dimerization: Pd (II) catalysis in the presence of oxygen using aqueous media
Parrish JP, et al.
Tetrahedron Letters, 43(44), 7899-7902 (2002)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)