推荐产品
品質等級
化驗
97%
形狀
solid
bp
133-135 °C/14 mmHg (lit.)
mp
35-39 °C (lit.)
密度
1.101 g/mL at 25 °C (lit.)
官能基
carboxylic acid
SMILES 字串
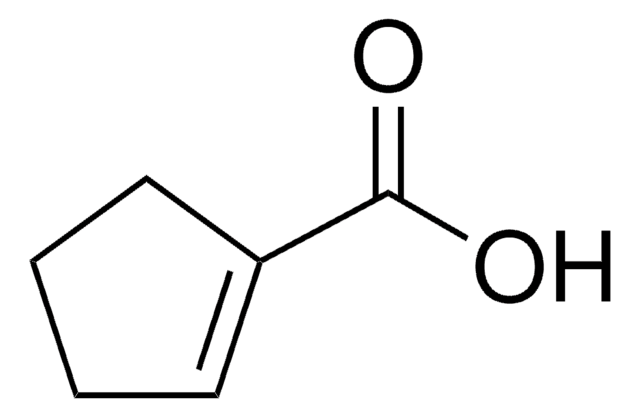
OC(=O)C1=CCCCC1
InChI
1S/C7H10O2/c8-7(9)6-4-2-1-3-5-6/h4H,1-3,5H2,(H,8,9)
InChI 密鑰
NMEZJSDUZQOPFE-UHFFFAOYSA-N
一般說明
在产甲烷菌无氧分解苯甲酸的过程中,1-环己烯-1-羧酸确定为中间体。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Mariana G de Oliveira et al.
American journal of physiology. Renal physiology, 315(3), F460-F468 (2018-05-03)
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a chronic inflammatory disease without consistently effective treatment. We investigate the role of toll-like receptor 4 (TLR4) on voiding dysfunction and inflammation in the cyclophosphamide (CYP)-induced mouse cystitis. Male C57BL/6 [wild-type, (WT)] and/or TLR4
Xin Xie et al.
Carbohydrate polymers, 225, 115223-115223 (2019-09-16)
A polysaccharide isolated from Strongylocentrotus nudus eggs (SEP) reportedly displays immune activity in vivo. Here, its effect and underlying mechanism in the treatment of pancreatic cancer were investigated. SEP obviously inhibited pancreatic cancer growth by activating NK cells in vitro/vivo
K A Reynolds et al.
Journal of bacteriology, 174(12), 3850-3854 (1992-06-01)
A novel NADPH-dependent enoyl reductase, catalyzing the conversion of 1-cyclohexenylcarbonyl coenzyme A (1-cyclohexenylcarbonyl-CoA) to cyclohexylcarbonyl-CoA, was purified to homogeneity from Streptomyces collinus. This enzyme, a dimer with subunits of identical M(r) (36,000), exhibits a Km of 1.5 +/- 0.3 microM
C L Keith et al.
Archives of microbiology, 118(2), 173-176 (1978-08-01)
A possible pathway for the anaerobic utilization of benzoic acid by a methanogenic consortium is suggested. Cyclohexane carboxylic acid and 1-cyclohexene-1-carboxylic acid have been identified as intermediates before ring rupture. Suprisingly, 3-cyclohexene-1-carboxylic acid interferes with utilization of other cyclic acids.
M S Elshahed et al.
Applied and environmental microbiology, 67(4), 1728-1738 (2001-04-03)
The metabolism of benzoate, cyclohex-1-ene carboxylate, and cyclohexane carboxylate by "Syntrophus aciditrophicus" in cocultures with hydrogen-using microorganisms was studied. Cyclohexane carboxylate, cyclohex-1-ene carboxylate, pimelate, and glutarate (or their coenzyme A [CoA] derivatives) transiently accumulated during growth with benzoate. Identification was
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持