推荐产品
化驗
98%
形狀
solid
折射率
n20/D 1.461 (lit.)
bp
232-233 °C (lit.)
mp
29-31 °C (lit.)
溶解度
H2O: soluble 0.201g in 100g at 15 °C
organic solvents: soluble
密度
1.033 g/mL at 25 °C (lit.)
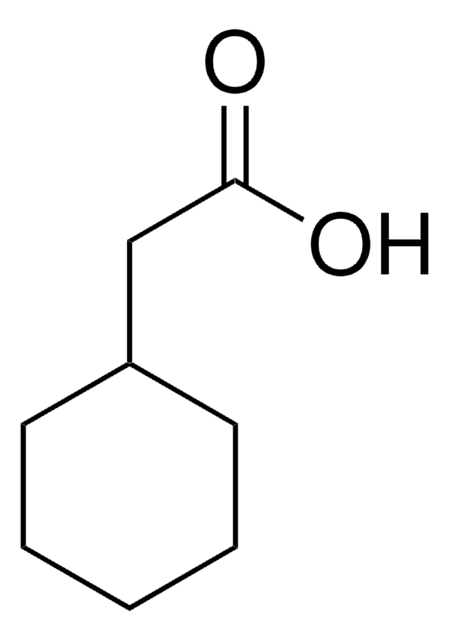
SMILES 字串
OC(=O)C1CCCCC1
InChI
1S/C7H12O2/c8-7(9)6-4-2-1-3-5-6/h6H,1-5H2,(H,8,9)
InChI 密鑰
NZNMSOFKMUBTKW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
环己烷羧酸被用于测定三种天然环糊精与七种环己烷衍生物的复合物结合常数 。
生化/生理作用
环己烷羧酸在一株厌氧杆菌的作用下发生微生物降解形成对羟基苯甲酸。环己烷羧酸发生芳构化,并在体外大鼠肝脏提取物中转化为马尿酸。环己烷羧酸是合成聚酮类抗生素——磷霉素的起始试剂。
準備報告
0.201g 环己烷羧酸溶于 15°C 100g 水中。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Biosynthesis of phoslactomycins: cyclohexanecarboxylic acid as the starter unit.
Sekiyama Y, et al.
Tetrahedron, 59(38), 7465-7471 (2003)
A Gadre et al.
Journal of pharmaceutical sciences, 86(2), 236-243 (1997-02-01)
Complex binding constants of the three native cyclodextrins with seven cyclohexane derivatives (all possessing the carboxylic acid group) and with the series C6H5(CH2)nCOOH (n = 0 to 4) were measured in aqueous solution at 25 degrees C by potentiometry and
The microbial degradation of cyclohexanecarboxylic acid: a pathway involving aromatization to form p-hydroxybenzoic acid.
Blakley ER.
Canadian Journal of Microbiology, 20(10), 1297-1306 (1974)
Christo Christov et al.
ChemMedChem, 6(1), 131-140 (2010-12-07)
2-(3,5-Dichlorophenylcarbamoyl)cyclohexanecarboxylic acid (1) is a potent and selective positive allosteric modulator of metabotropic glutamate receptor subtype 4 (mGluR4). The activity of 1 was reported to reside in the cis diastereomer with equal potency between its enantiomeric forms (Niswender et al., Mol. Pharmacol.
P G Egland et al.
Journal of bacteriology, 177(22), 6545-6551 (1995-11-01)
The first step of anaerobic benzoate degradation is the formation of benzoyl-coenzyme A by benzoate-coenzyme A ligase. This enzyme, purified from Rhodopseudomonas palustris, is maximally active with 5 microM benzoate. To study the molecular basis for this reaction, the benzoate-coenzyme
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门