推荐产品
蒸汽密度
4.4 (vs air)
质量水平
蒸汽压
150 mmHg ( 20 °C)
产品线
ReagentPlus®
方案
≥99%
表单
liquid
反应适用性
reagent type: oxidant
杂质
<10 ppb Heavy metals
颜色
APHA: 0-150
折射率
n20/D 1.429 (lit.)
沸点
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
密度
1.5 g/mL at 20 °C (lit.)
官能团
acyl chloride
SMILES字符串
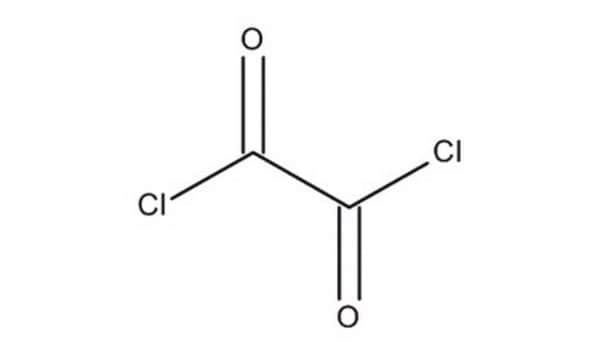
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般描述
草酰氯是一种常用的氯化试剂,可以通过草酸和五氯化磷反应制备。
应用
作为以下反应的反应物:
- N-杂环炔酮和炔酮合成,用于活化羧酸
- 氯化和卤化
- 三组分 [3+2]环加成
- 有机锡反应
- 环戊烯酮合成
- 羰基化,用作羰基合成子
可以用来合成用于生产液体结晶的酸性氯化物。
草酰氯可以用于以下反应:
- 在DMF存在下,酸性氯化物和Mosher′s酸反应制备Mosher
- 活化二甲亚砜,用于长链酒精生产羰基的氧化。
- 活化α-酮羧酸和N杂环羧酸,分别炔基化形成炔二酮和N杂环炔酮。
包装
在美国售卖的5g、25g和100g规格以安瓿瓶包装。
法律信息
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
警示用语:
Danger
危险分类
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
补充剂危害
储存分类代码
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 1
闪点(°F)
51.8 °F - closed cup
闪点(°C)
11.0 °C - closed cup
个人防护装备
Faceshields, Gloves, Goggles
其他客户在看
Catalytic Syntheses of N-Heterocyclic Ynones and Ynediones by In Situ Activation of Carboxylic Acids with Oxalyl Chloride.
Boersch C, et al.
Angewandte Chemie (International Edition in English), 50(44), 10448-10452 (2011)
A simple method for the microscale preparation of Mosher's acid chloride.
Ward DE and Rhee CK.
Tetrahedron Letters, 32(49), 7165-7166 (1991)
Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide" activated" by oxalyl chloride.
Mancuso AJ, et al.
The Journal of Organic Chemistry, 43(12), 2480-2482 (1978)
Tsutomu Kimura et al.
Chemical communications (Cambridge, England), (32)(32), 4077-4079 (2005-08-11)
Reaction of diastereomerically pure phosphinoselenoic acid salts with oxalyl chloride leads to enantiomerically pure P-chiral phosphinoselenoic chlorides with inversion of configuration at phosphorus; one of these chlorides is converted to a phosphinoselenothioic acid salt with a high degree of enantioselectivity.
Peter J Manley et al.
Organic letters, 4(18), 3127-3129 (2002-08-31)
[reaction: see text] A mild, practical, one-pot method for the generation of imidoyl chlorides and their subsequent in situ reaction with pyridine-1-oxides is described. The imidoyl chlorides were formed from the reaction of secondary amides with a stoichiometric amount of
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持