推荐产品
product name
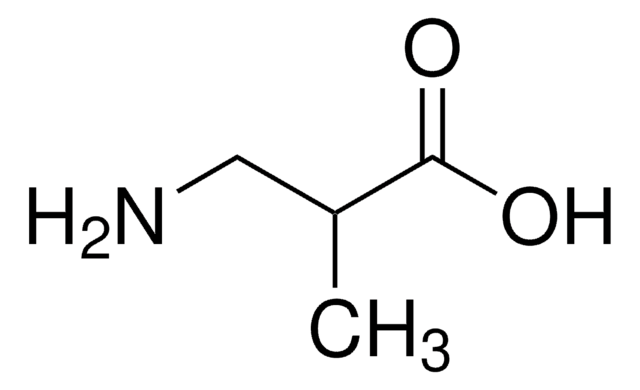
DL -2-氨基丁酸, ReagentPlus®, 99%
品質等級
產品線
ReagentPlus®
化驗
99%
形狀
solid
反應適用性
reaction type: solution phase peptide synthesis
mp
291 °C (dec.) (lit.)
應用
peptide synthesis
SMILES 字串
CCC(N)C(O)=O
InChI
1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)
InChI 密鑰
QWCKQJZIFLGMSD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
DL-2-氨基丁酸,又称为α-氨基丁酸,常用于液相多肽合成法。
應用
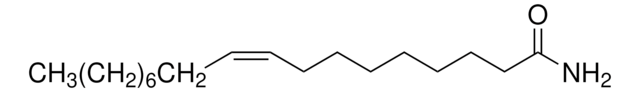
DL-2-氨基丁酸可用于合成2-(2,5-二氧代吡咯烷-1-基)丁酸。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Design, synthesis, and anticonvulsant activity of new hybrid compounds derived from 2-(2, 5-dioxopyrrolidin-1-yl) propanamides and 2-(2, 5-dioxopyrrolidin-1-yl) butanamides
Journal of Medicinal Chemistry, 58, 5274-5286 (2015)
Ryan J Martinie et al.
Journal of the American Chemical Society, 137(21), 6912-6919 (2015-05-13)
The iron(II)- and 2-(oxo)glutarate-dependent (Fe/2OG) oxygenases catalyze an array of challenging transformations, but how individual members of the enzyme family direct different outcomes is poorly understood. The Fe/2OG halogenase, SyrB2, chlorinates C4 of its native substrate, l-threonine appended to the
Izumi Kawabata et al.
Nature communications, 3, 722-722 (2012-03-08)
Synaptic remodelling coordinated with dendritic growth is essential for proper development of neural connections. After establishment of synaptic contacts, synaptic junctions are thought to become stationary and provide fixed anchoring points for further dendritic growth. However, the possibility of active
Danica P Galonić et al.
Nature chemical biology, 3(2), 113-116 (2007-01-16)
Enzymatic incorporation of a halogen atom is a common feature in the biosyntheses of more than 4,500 natural products. Halogenation of unactivated carbon centers in the biosyntheses of several compounds of nonribosomal peptide origin is carried out by a class
Young-Man Seo et al.
Organic & biomolecular chemistry, 10(12), 2482-2485 (2012-02-22)
A deracemization method was developed to generate optically pure L-homoalanine from racemic homoalanine using D-amino acid oxidase and ω-transaminase. A whole cell reaction using a biphasic system converted 500 mM racemic homoalanine to 485 mM L-homoalanine (>99% ee).
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门